See also: Bartonella: Not a Lyme coinfection, many false diagnoses
LymeScience republishes:
Demographic characteristics of teenage boys with horizontal striae distensae of the lower back
Emily Boozalis, BA | Anna L. Grossberg, MD | Katherine B. Puttgen, MD | Candrice R. Heath MD | Bernard A. Cohen, MD
Department of Dermatology, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
First published November 21, 2017 by the Wiley journal Pediatric Dermatology.
Abstract
Background
This study examines the clinical characteristics and demographics of teenage boys with horizontal striae distensae of the lower back in an outpatient setting.
Methods
Retrospective medical chart reviews and telephone survey studies were completed on an outpatient cohort of 12 boys 11 to 17 years of age with a clinical diagnosis of transverse striae distensae of the lower back at a single-center, university-based, pediatric dermatology practice. We evaluated the clinical features of the striae, participant demographic characteristics, and past medical history. A review of the literature concerning risk factors was conducted using PubMed and Google Scholar.
Results
Of the 14 patients we contacted, 12 agreed to participate. The average age of onset for the striae was 14.3 years. All boys were above the 50th percentile in height at the time of onset. Eight (66.7%) reported a significant growth spurt before the appearance of the stretch marks. Most were asymptomatic. None of the boys had a history of unmonitored exogenous steroid use or prior infection with Bartonella henselae or Borrelia burgdorferi. Only one (8.3%) had a chronic medical condition. Eleven (91.7%) had at least one first-degree relative with striae distensae.
Conclusion
Our results indicate that horizontal striae distensae of the lower back in adolescent boys is associated with a rapid growth spurt, tall stature, and family history of striae distensae. There is no association between this type of striae distensae and any chronic medical condition, bacterial infection, or exogenous steroid use. Thus a careful review of systems and counseling without further medical testing is reasonable management.
1 INTRODUCTION
Striae distensae (SD, striae, stretch marks) are common linear atrophic plaques that can cause cosmetic concern.1 Although striae occur in up to 86% of adolescents, few studies have evaluated the demographic characteristics or risk factors associated with this clinical finding.2 We were specifically interested in a subset of individuals with SD: boys 11 to 17 years of age with horizontal striae of the lower back. Common concerns that these boys were using unmonitored exogenous steroids, had a metabolic condition, or were infected with Bartonella henselae (cat-scratch disease) or Borrelia burgdorferi (Lyme disease) prompted our study.3–5 This specific subset of the population with SD has not been previously evaluated. Thus we performed telephone surveys and retrospective medical chart reviews of 12 teenage boys with horizontal SD of the lower back to evaluate demographic factors and comorbidities associated with this condition. We also reviewed the current literature.
2 PARTICIPANTS AND METHODS
2.1 Study Design
The Johns Hopkins Institutional Review Board approved the study protocol, which was a single-center, retrospective survey study evaluating clinical characteristics, demographic data, and potential risk factors in teenage boys with a clinical diagnosis of transverse SD of the lower back. Telephone surveys and retrospective medical chart reviews were conducted at the Johns Hopkins Pediatric Dermatology Clinic in Baltimore, Maryland, between June 2016 and December 2016.
2.2 Participants and Survey Methods
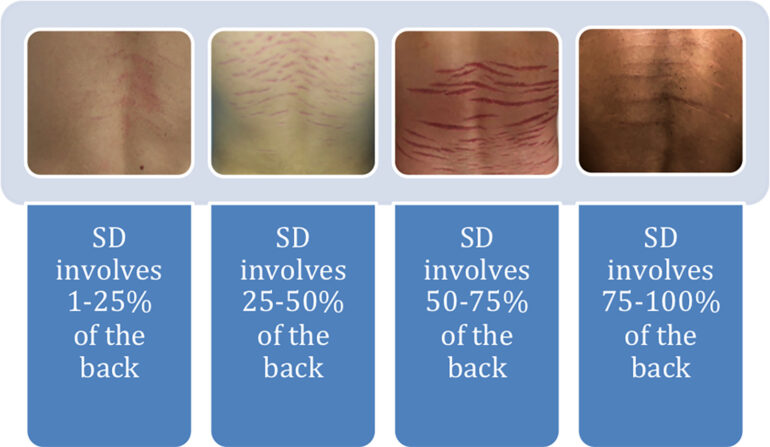
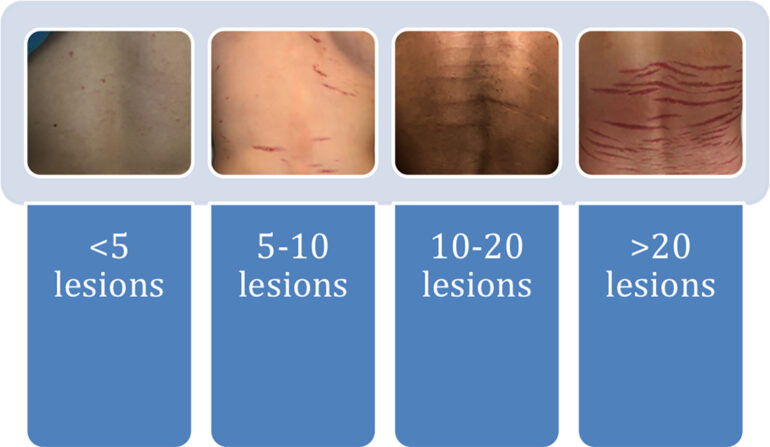
Eligible individuals were boys younger than 18 years at the time of clinical diagnosis of horizontal SD of the lower back, as assessed by a pediatric dermatologist at the Johns Hopkins Pediatric Dermatology Clinic between January 2016 and December 2016. Patients presented with horizontal SD as their primary complaint or were found to have striae incidentally and were recruited for the study. Qualifying patients whose parents agreed to participate in the study at the time of their clinic visit were contacted for telephone interviews. Telephone interviews were conducted to determine race, age of onset, height and weight at the time of onset, presence of striae in any other locations, past history of rapid growth spurts, history of acne, and family history of striae. We also evaluated potential causative factors, including unmonitored injectable or oral anabolic steroid use, chronic medical conditions, and prior infection with B. henselae or B. burgdorferi. We called each patient’s household at various times of day and on different days to optimize contact with the patient. If the patient was a minor at the time of the interview, the initial respondent acted as a surrogate and completed the interview on his behalf. Height and weight percentiles were calculated using the Centers for Disease Control and Prevention growth charts for boys 2 years of age and older. We photographed each patient’s SD and assessed the degree of involvement, number of lesions, and extent of erythema using a visual scoring system (Figures 1 and 2).
3 RESULTS
3.1 Participants
Of 14 identified adolescent boys with transverse SD of the lower back, 12 (85.7%) participated in the study. Those who did not participate could not be reached or chose not to participate. Two (16.7%) of the patients contacted were African American, nine (75%) were Caucasian, and one (8.3%) was Asian. The average age of onset of SD was 14.3 y (range 11–17). All 12 of the boys with horizontal SD of the lower back were above the 50th percentile in height at the time of onset, with an average height percentile of 88.1%; 58.3% were above the 50th percentile for weight when the striae first appeared, with an average weight percentile of 64%. The average body mass index (BMI) was 19.8 kg/m2 (range 12.5-33), with six (50%) underweight and only one obese (8.3%). Eight patients (66.7%) reported a noticeable growth spurt before striae onset. Most striae were asymptomatic (83.3%). The only symptom reported in association with the striae was pruritus. Four patients (33.3%) presented with striae in other locations, most commonly the inner thighs and posterior shoulders. Nine (75%) also had acne, most commonly on the forehead. None had a history of unmonitored exogenous anabolic steroid use or bacterial infection with B. henselae or B. burgdorferi. Only one (8.3%) had a chronic medical condition, Peutz–Jeghers syndrome. Almost all had at least one first-degree relative with stretch marks. Demographic and medical history characteristics are presented in Table 1.
Table 1. Participant demographic characteristics (N = 12)
| Characteristic | Value |
|---|---|
| Age, average | 14.3 |
| Race, n (%) | |
| Caucasian | 9 (75) |
| African American | 2 (16.7) |
| Asian | 1 (8.3) |
| Average height percentile, % | 88.1 |
| Above 50th percentile, n (%) | 12 (100) |
| Below 50th percentile, n (%) | 0 (0) |
| Average weight percentile, % | 64 |
| Above 50th percentile, n (%) | 7 (58.3) |
| Below 50th percentile, n (%) | 5 (41.7) |
| Body mass index, average | 19.8 (12.5—33) |
| Underweight, n (%) | 6 (50) |
| Normal weight, n (%) | 5 (41.7) |
| Obese, n (%) | 1 (8.3) |
| Associated pruritus, n (%) | 2 (16.7) |
| Striae in other locations, n (%) | 4 (33.3) |
| Inner thighs | 3 (25) |
| Posterior shoulders | 2 (16.7) |
| Axillae | 1 (8.3) |
| Biceps | 1 (8.3) |
| Growth spurt before striae onset, n (%) | 8 (66.7) |
| History of acne, n (%) | 9 (75) |
| Face | 9 (75) |
| Back | 4 (33.3) |
| Prior unmonitored steroid use, n (%) | 0 (0) |
| Underlying chronic medical conditions, n (%)a | 1 (8.3) |
| Infection with Bartonella henselae, n (%) | 0 (0) |
| Infection with Borrelia burgdorferi, n (%) | 0 (0) |
| First-degree relative with stretch marks, n (%) | 11 (91.7) |
a Peutz–Jeghers syndrome.
3.2 Transverse SD Characteristics
Seven (58.3%) had more than 10 lesions at the time of diagnosis. Most striae involved less than half of the back. The extent of erythema varied based on the stage of the striae. Three patients (25%) presented with striae rubrae, or the initial, erythematous stage of SD, and five (41.7%) presented with striae albae, or the white, later stage of SD. Striae characteristics are shown in Table 2.
Table 2. Striae distensae characteristics
| Characteristic | n (%) |
|---|---|
| Number of lesions at diagnosis | |
| <5 | 1 (8.3) |
| 5-10 | 4 (33.3) |
| 10-20 | 3 (25) |
| >20 | 4 (33.3) |
| Extent of involvement of the back, % | |
| 0-25 | 3 (25) |
| 25-50 | 5 (41.7) |
| 50-75 | 1 (8.3) |
| 75-100 | 3 (25) |
4 DISCUSSION
Striae distensae are linear atrophic plaques characterized by epidermal thinning that can cause significant psychological burden to the patient. Although the exact pathogenesis is poorly understood, striae are thought to occur due to mechanical stretching of the skin, hormonal changes, or as a result of innate structural disturbances in the integument.1 In adolescents, clinical findings such as high BMI, obesity during childhood, and facial seborrhea correlate positively with development of SD.1, 2 Striae have also been observed in monozygotic twins and in conjunction with Cushing syndrome, exogenous steroid use, and Marfan syndrome.2 The prevalence of SD in adolescents that has been reported in the literature varies significantly (range 6-86%).1 Despite SD being common, contributing factors are not well understood.
We focused on a subset of adolescents with SD: boys 11 to 17 years of age with horizontal SD of the lower back. The decision to focus on this subset of patients stemmed from increasingly common concerns of parents and health care providers that these boys are taking unmonitored injectable or oral steroids, have an underlying metabolic condition such as Cushing syndrome, or are infected with B. henselae or B. burgdorferi. There are two instances in the literature in which this type of striae was mistaken for child abuse.6, 7
We found that none of the boys with horizontal SD of the lower back had a history of unmonitored injectable or oral anabolic steroid use. Although roughly one-third of boys and men using noncritical androgenic anabolic substances have associated SD, the striae are most consistently reported in the axillary and deltopectoral regions rather than across the lower back.8, 9 Primary care providers can use our data to help dispel speculations that boys with striae of the lower back are using unmonitored exogenous steroids.
Another common concern of parents is that horizontal SD of the lower back indicates Cushing syndrome or another metabolic condition. If a patient with transverse SD has a BMI in the normal range, metabolic syndrome is unlikely to be an underlying problem. None of our patients with SD of the lower back reported a history of metabolic disease. Only one had a chronic medical condition, Peutz–Jeghers syndrome. According to the literature, 71.4% of boys with Cushing syndrome have wide, violaceous striae in the abdominal or gluteal region,10 but there have been no reports of horizontal SD of the lower back in conjunction with this diagnosis. Thus there is no evidence in the literature or from our study that supports the need for evaluation for chronic metabolic conditions in an individual whose only symptom is horizontal SD of the lower back.
Finally, there is growing concern among children and their parents that striae of the lower back are a symptom of infection with B. henselae or B. burgdorferi. None of the patients in our study reported prior infection with either of these organisms. We could not find any evidence in the literature to support an association between Lyme disease and development of SD. There is only one case report of a teenage boy (age 18y) with cat-scratch disease who presented with striae as a possible symptom of the disease. According to the report, the striae appeared on both thighs and the buttocks; blood serology and a biopsy of the striae revealed a diagnosis of bartonellosis.11 Although that study provided one case report suggesting striae as a potential symptom of cat-scratch disease, no one has investigated or confirmed this association, but many lay publications and online forums cite coinfection of Lyme disease with cat-scratch disease as a cause of striae, which probably perpetuates these concerns in parents.3–5, 12, 13 According to websites such as lymedisease.org, lymeactionnetwork.org, and lymeneteurope.org, ticks can coinfect people with Lyme disease and cat-scratch disease, leading to the development of a rash resembling stretch marks.3–5 No medical organizations back any of these websites, nor do the websites cite any published medical literature supporting the accuracy of their information. Although scientific studies have found tick bites to be a risk factor for infection with Bartonella species, the capacity for ticks to transmit B. henselae is the subject of substantial debate.14 It is important for primary care providers to be aware of these online forums, because they often underlie parental concerns. Physicians can use our findings and review of the literature to prevent unnecessary medical testing and reassure patients that there is no association between stretch marks of the lumbosacral region and infection with either of these pathogens.
4.1 Limitations
This is the first clinical study assessing the demographic characteristics of teenage boys with horizontal striae of the lower back. Additional studies with larger sample sizes are warranted before definitive conclusions can be drawn.
This was a retrospective study, which creates the potential for recall bias. Individuals of high socioeconomic status may have been overrepresented, because families with multiple telephones were more likely to be contacted for the survey. Titers for B. henselae and borreliosis were only determined in one of the patients before he was referred to pediatric dermatology, which limits our ability to exclude the possibility of infection as the trigger for SD.
4.2 Conclusion
Overall, our study found that horizontal SD of the lower back in adolescent boys is associated with rapid growth spurts, tall stature, and a positive family history of SD. These findings suggest that the striae are a normal physiological process in growing adolescent boys rather than a sign of underlying pathology. Larger controlled studies with long-term follow-up are necessary to confirm our findings and to more precisely assess the factors that influence the development of SD in teenage boys.
- Al-Himdani S, Ud-Din S, Gilmore S, et al. Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol. 2014;170:527-547.
- Cho S, Park ES, Lee DH, et al. Clinical features and risk factors for striae distensae in Korean adolescents. J Eur Acad Dermatol Venereol. 2006; 20: 1108- 1113.
- Lyme disease: Bartonella [online]. Accessed June 22, 2016.
- Lyme Action Network. Facts, Co-Infections, Symptoms [online]. Accessed June 22, 2016.
- Bartonella vs. Stretch Marks & Other Rashes/Skin Problems [online]. Accessed June 22, 2016.
- Masand M. Physiological striae in adolescence: not physical abuse. Emerg Med J. 2012; 29: 9.
- Burk C, Pandrangi B, Connelly E. Picture of the month—diagnosis. Arch Pediatr Adolesc Med. 2008; 162: 227.
- Wollina U, Pabst F, Schonlebe J, et al. Side-effects of topical androgenic and anabolic substances and steroids. A short review. Acta Dermatovenerol Alp Pannonica Adriat. 2007; 16: 117- 122.
- Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997; 31: 54- 58.
- Libuit LG, Karageorgiadis AS, Sinaii N, et al. A gender-dependent analysis of Cushing’s disease in childhood: pre- and postoperative follow-up. Clin Endocrinol (Oxf). 2015; 83: 72- 77.
- Maggi RG, Ericson M, Mascarelli PE, et al. Bartonella henselae bacteremia in a mother and son potentially associated with tick exposure. Parasit Vectors. 2013; 6: 101.
- Schaller JL. Bartonella: Diagnosis and Treatment, vol. 1. Tampa, Florida: Hope Academic Press; 2008.
- Kohlstadt I. Advancing Medicine with Food and Nutrients, vol. 1. New York: CRC Press; 2012.
- Diniz PP, Velho PE, Pitassi LH, et al. Risk factors for Bartonella species infection in blood donors from southeast Brazil. PLoS Negl Trop Dis. 2016;10:e0004509.
This is the peer reviewed version of the following article: Boozalis E, Grossberg AL, Puttgen KB, Heath CR, Cohen BA. Demographic characteristics of teenage boys with horizontal striae distensae of the lower back. Pediatr Dermatol. 2018 Jan;35(1):59-63. doi: 10.1111/pde.13329. Epub 2017 Nov 21., which has been published in final form at the journal Pediatric Dermatology.
This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Use of Self-Archived Versions. This article may not be enhanced, enriched or otherwise transformed into a derivative work, without express permission from Wiley or by statutory rights under applicable legislation. Copyright notices must not be removed, obscured or modified. The article must be linked to Wiley’s version of record on Wiley Online Library and any embedding, framing or otherwise making available the article or pages thereof by third parties from platforms, services and websites other than Wiley Online Library must be prohibited.
The above article has been adapted for internet display by the LymeScience Document Repository and posted with author permission.