In the United States, there is a class of testing called “Laboratory-developed tests” (LDTs) that has little regulation from the FDA or other government health authorities.
Peddlers of fraudulent LDTs—like Theranos—have exploited this lack of regulation to profit while endangering patients.
When it comes to the alternate universe of chronic Lyme pseudoscience, there is heavy demand for testing that will facilitate (fake) diagnoses. People want answers to their problems, and quacks who market themselves as functional, integrative, naturopathic, and “Lyme literate” are happy to sell false answers.
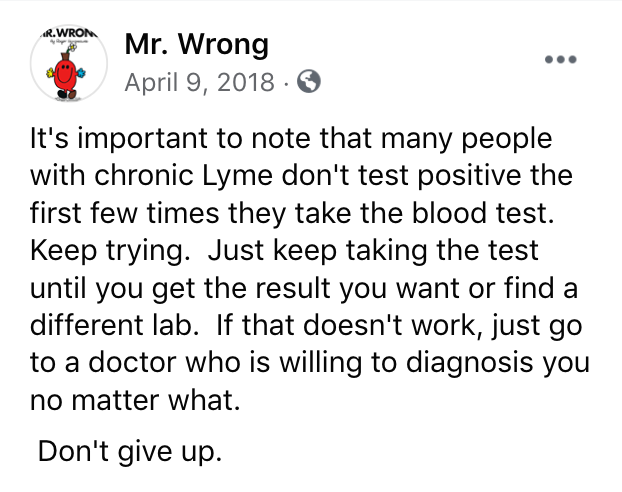
The satirical Mr. Wrong parodied the shopping for positive results that is encouraged in dangerous “support groups” online:
One 2014 study by Brian Fallon, MD (whose beliefs are contrary to established science and who has taken millions of dollars from pseudoscience advocates) found that samples from 23 of 40 healthy people could be considered positive according to immunoblot criteria promoted by the shady for-profit lab IgeneX.
IgeneX executives have had very close ties with the dangerous pseudoscience group ILADS (International Lyme and Associated Diseases Society). As noted by experts, “Lyme literate” doctors are notorious for their ethics violations, bizarre beliefs, and recommendations of IgeneX testing.
The long-time president and founder of IgeneX Nick Harris was also a founding leader (treasurer, maintaining the financials) of ILADS. Nick Harris co-authored ILADS’s discredited treatment guidelines, which failed to disclose his IgeneX affiliation. Nick Harris is the father of ILADS “Lyme literate” grifter and IgeneX employee Steven Harris, MD, who was disciplined for injecting patients with garlic. The laboratory director and later president of IgeneX Jyotsna S. Shah, PhD became a board member of the ILADS entity ILADEF. Steven Harris also became an ILADS board member.
ILADS charlatans have even used IgeneX testing to diagnose “chronic Lyme” in places where real Lyme disease is rare or non-existent. ILADS members like Dr. Peter Mayne in Australia—where there is no endemic Lyme disease—have used IgeneX testing to facilitate quack diagnoses. Mayne was found guilty of unsatisfactory professional conduct for his horrific treatment and inappropriate diagnosis of a patient, who died after a delayed cancer diagnosis.
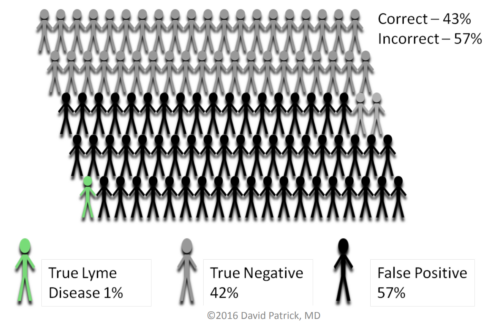
One member of a support group was horrified when they learned that potentially 57.5% of people could receive a false positive Lyme diagnosis.
David Patrick, MD, a researcher at University of British Columbia, explained the implications with a hypothetical population of 100 people, with one having Lyme disease. In this hypothetical, 57 might test falsely positive according to IgeneX criteria, 42 would receive a true negative, and one would receive a true positive test. This means that 57 of 58, or 98% of the positive test results would be false. This is a positive predictive value of 2%, a dismal result.
There are very good reasons that the CDC has long-warned about “using assays whose accuracy and clinical usefulness have not been adequately established”, like urine tests and in-house criteria for interpretation of immunoblots. The best bet for Lyme disease is to use—in the appropriate clinical context—CDC-recommended and FDA-cleared or approved testing, or the equivalent European tests.
In Canada, experts are also concerned about shady foreign laboratories like IgeneX. In Ontario, the fake doctors known as naturopaths were told to stop using IgeneX. And citing the Fallon study, the Nova Scotia Infectious Diseases Expert Group explicitly recommends against:
Sending specimens to laboratories that use interpretive criteria that are different from the CDC: the most common request that physicians get is to send the specimen to IGeneX, which has different interpretive criteria for their immunoblots, which may lead to false positive test results (Fallon et al., 2014; Molins et al., 2016).
Besides Lyme tests, IgeneX offers a large menu of dubious pricy tests, which are often used by the “Lyme literate” to justify “co-infection” diagnoses. ILADS conspiracy theorists John and Catherine Scott used predatory publisher MDPI to report 1119 alleged cases of Babesiosis caused by Babesia duncani in a seven year period from 2011 to 2017 from patients who lived all over Canada, where babesiosis is super rare. The diagnoses—a portion of which were based on IgeneX tests—were attributed to ten fake doctors (naturopaths) and ten physicians in a predatory “special issue” edited by former ILADS president Raphael Stricker, MD, who was fired by UCSF after being found guilty of scientific misconduct. Later, Stricker was found guilty of scientific misconduct by the NIH, which cited “falsified data” that was used to justify a taxpayer-funded grant.
Contrary to the unbelievable ILADS reports, in 2020, experts noted, “A small number of B. duncani cases (<15) have been reported on the West Coast of the United States.” And among even the rare reported Canadian babesiosis cases, we found no cases of B. duncani that were unconnected to ILADS. And despite the Scotts’s reports of a need for longer treatment and that “optimum antibabesiosis treatment for persistent B. duncani infection remains to be determined,” most reported B. duncani cases have been effectively treated with standard 7-10 day antimicrobial regimens. We also found no evidence to support the Scotts’s claim that B. duncani “is not limited to the Pacific Northwest.” Experts also emphasized that Babesia parasites can be directly detected by PCR and blood smear examination, and that antibody testing should not be the basis of diagnosis.
In 2015, the FDA published a report detailing 20 case studies of serious concerns about LDTs.
The conclusion and case study on Lyme disease from the report are reproduced below. The case study notes three infamous cases of discredited Lyme disease testing:
- IgeneX’s urine test, which was also called unreliable by the NIH. (This is a different test from the IgeneX immunoblot criteria discussed above.)
- Testing facilitated by “Lyme literate” fraudsters Carol Ryser, MD and JoAnne Whitaker, MD.
- A culture test by Advanced Laboratory Services, Inc. Long-time charlatans Joseph Burrascano, MD and Eva Sapi, PhD were both financially connected to this scandal. According to an odd statement by Medscape, “Dr. Burrascano has disclosed no financial interest in the laboratory, in the Borrelia culture, or in any intellectual property and receives no commissions from the tests. Dr. Burrascano is senior vice president of medical affairs and medical director for Advanced Research Corporation, a contract research organization with the same president and corporate address as Advanced Laboratory Services, Inc.” He was also a “Lyme literate” founding leader (first vice president) of ILADS. In the 2000s, Burrascano was found guilty of negligence and professional misconduct. After the Advanced Laboratory debacle, Burrascano became an employee of IgeneX.
Case Studies of Problematic LDTs
A. Tests that Yield Many Positive Results when the Disease or Condition is not Actually Present (False-Positives)
i. Lyme Disease Diagnostic Tests
| Category | LDT Characteristics |
|---|---|
| LDT Name | Lyme disease antigen and culture tests |
| Description | Test to detect portions of the bacterium that causes Lyme disease or antibodies to the bacterium |
| Purpose | Diagnose Lyme disease |
| Target Population | Patients with symptoms suggestive of Lyme disease |
| Alternatives | Over 80 FDA-cleared diagnostic tests |
| LDT Problem 1 | In clinical use, large numbers of patients with positive tests do not have Lyme disease |
| Clinical Consequence | Patients with false-positive tests may be treated with unnecessary medications; delayed diagnosis of true underlying condition |
| Potential Impact of FDA Oversight | Assurance the test meets minimum performance standards |
| Cost Impact of Inaccuracy | $1,226 per case |
Lyme disease is caused by infection with the bacterium Borrelia burgdorferi, transmitted to humans by the bite of an infected tick. The diagnosis is based on a history of exposure to ticks along with typical symptoms, including fever, fatigue, muscle, and joint aches, and a characteristic rash.4 CDC recommends a two–test process to detect antibodies against B. burgdorferi.5 If an initial enzyme-linked immunosorbent assay test is positive or indeterminate, it is followed by a confirmatory Western Blot test.
A patient is only diagnosed with Lyme disease if the confirmatory Western Blot is positive. As of May 2015, over 80 initial and confirmatory diagnostic blood tests for Lyme disease had been cleared by FDA.6 Patients diagnosed with Lyme disease are treated with oral or intravenous antibiotics for 2-4 weeks. This relieves symptoms in 80%-90% of patients, but can lead to harmful side effects, including nausea, allergic reactions,7 and intravenous site infection.8 A falsely positive diagnosis of Lyme disease can lead to patients experiencing harmful side effects without clinical benefit, an increase in the risk of creating infectious organisms resistant to the antibiotics used to treat Lyme disease, and delay in the diagnosis of a patient’s true underlying condition.
Between 2000 and 2005, a “Dot Blot” test for urine antigens against Lyme disease was offered, claiming a 97% “true positive rate,” although this term does not have a clear meaning in public health terms.9 An independent evaluation conducted in 2001 ran the test five times for the same10 healthy subjects (i.e., 50 tests) and found that the test was consistently falsely positive in all tests run for two subjects (10 false-positive tests) and gave contradictory results on at least two pairs of tests for 8 subjects (i.e., at least 16 false-positive tests), leading to the conclusion that at least half of all test results were incorrect or uninterpretable, and that this test should not be used for Lyme disease detection.9
Further research also indicated that, because of lack of a clear correlation with clinical disease, urine tests in general are not appropriate for the diagnosis of Lyme disease,10 but sales continued with between 50,000 and 70,000 tests sold in 2005.11 Early that year, however, the LDT was implicated in 8 reports of false-positive diagnoses.11
Diagnostic tests for Lyme antigens in the blood also have been marketed. One marketed between 2003 and 2005 was prone to false-positives. On the basis of false-positive results, two couples underwent months of unnecessary treatment with antibiotics and other alternative medications.12 After litigation, a judge awarded them a total of $30 million in damages.12
In April 2014, CDC issued a warning related to a Lyme disease culture test.13 The Agency had conducted a review14 that “raised serious concerns about false-positive results caused by laboratory contamination and the potential for misdiagnosis.” Consequently, CDC recommended that only FDA-cleared/approved diagnostics for Lyme disease be used.
FDA estimated the cost of a false-positive diagnosis as the direct medical treatment costs for a patient with early-stage Lyme disease. The most relevant and comprehensive estimate (in Year 2000 dollars) comes from a study of Lyme disease patients with varying severities of disease and includes the costs of health care provider visits, consultation, serologic testing, therapy, hospitalization, and out-of-pocket costs of prescription and non-prescription drugs.15 Updating the mean per-patient costs to current dollars using the medical care component of the Consumer Price Index yields an estimate of $1,22616 for the cost to society for each case.17
Conclusion
In this report, we have reviewed events related to 20 LDTs in which patients have been demonstrably harmed or may have been harmed by tests that did not meet FDA requirements. Tests that are inaccurate, unreliable, or have unproven or disproven claims expose patients to a range of harms. These include patients told incorrectly that they have life-threatening diseases and others whose lifethreatening diseases have gone undetected.
Despite arguments from some that “CLIA is enough,” all of the tests described as problematic in this report were offered from laboratories following the minimum requirements of CLIA. Specifically, CLIA does not:
- Ensure the safety and effectiveness of LDTs prior to marketing.
- Assess the quality of the design and manufacture of devices.
- Ensure test labeling provides adequate directions for use.
- Require truth in marketing materials and other labeling.
- Require adverse event reporting.
- Permit removal of unsafe devices from the market.
- Require informed consent for patients participating in clinical studies of LDTs.
- Establish procedures for the conduct of such studies.
These cases, therefore, highlight the need for greater FDA oversight of LDTs that is appropriately tailored so that it is complementary and does not duplicate the oversight currently provided under CLIA. Greater FDA oversight is needed to promote access to LDTs that provide benefits to patients and the health care system, while helping to ensure patients are not unduly exposed to harm.
References:
FDA: The Public Health Evidence for FDA Oversight of Laboratory Developed Tests: 20 Case Studies
LymeScience: NIH discusses IgeneX unreliability for Lyme testing
LymeScience: UK warns about inappropriate Lyme disease testing
Nova Scotia Infectious Diseases Expert Group: Guidance for Primary Care and Emergency Medicine Providers in the Management of Lyme Disease in Nova Scotia
CDC: Laboratory tests that are not recommended
Auwaerter PG, et al. Antiscience and ethical concerns associated with advocacy of Lyme disease. Lancet Infect Dis. 2011;11(9):713-9.
Jotwani R, et al. Theranos Experience Exposes Weaknesses in FDA Regulatory Discretion. Clin Pharmacol Drug Dev. 2017.
The Verge: FDA wants to close the loophole that Theranos used, but Republicans don’t understand why, 2015-11-17
The Verge: Theranos is gone, but ads for shady tests aren’t, 2021-12-06
Medscape: Lyme Culture Test Causes Uproar, 2013-01-30
Quackwatch: Some Notes on Nonstandard Lyme Disease Tests
Ana Santos Rutschman: How Theranos’ faulty blood tests got to market – and what that shows about gaps in FDA regulation, 2021-10-05
Stephanie Rogus, PhD, RDN, and Peter G. Lurie, MD, MPH: FDA Is Letting Harmful Lab-Developed Tests Fall Through the Cracks
Pew Research: The Role of Lab-Developed Tests in the In Vitro Diagnostics Market, 2021-10-22
Science-Based Medicine: Lemons and Lyme: Bogus tests and dangerous treatments of the Lyme-literati, 2014-07-18
Science-Based Medicine: New FDA regulatory role threatens bogus diagnostic tests, 2015-01-15
Science-Based Medicine: Lyme Testing, 2016-01-08
Science-Based Medicine: Experts slam CAM lab tests, call for better regulation, 2019-03-14
Science-Based Medicine: Too many lab tests still escape FDA review, threatening patient safety, 2021-11-04
Johnson BJ, Pilgard MA, Russell TM. Reply to “No evidence for contamination of Borrelia blood cultures: a review of facts”. J Clin Microbiol. 2014.
References from the above excerpt from the FDA report
4. Centers for Disease Control and Prevention. Signs and symptoms of Lyme disease. April 12, 2011. Accessed November 16, 2014.
5. Centers for Disease Control and Prevention. Two-step laboratory testing process. April 12, 2011. Accessed November 16, 2014.
6. U.S. Food and Drug Administration. Devices at FDA. December 8, 2014. Accessed December 1, 2014.
7. Wormser GP, Dattwyler RJ, Shapiro ED. The clinical assessment, treatment, and prevention of Lyme disease, Human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089-134.
8. Centers for Disease Control and Prevention. Lyme disease Treatment. April 12, 2011. Accessed November 14, 2014.
9. Klempner MS, Schmid CH, Hu L, et al. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am J Medicine. 2001;110(3):217-9. [LymeScience discussion]
10. Rauter C, Mueller M, Diterich I, et al. Critical evaluation of urine-based PCR assay for diagnosis of Lyme borreliosis. Clin Diagn Lab Immunol. 2005;12(8):910–7.
11. Hurley D, Santora M. Unproved Lyme disease tests prompt warnings. New York Times. August 23, 2005. Accessed November 14, 2014. (paywall-free link)
12. Margolies D. Who’s left to pay this big verdict? Kansas City Star, May 25, 2009. Accessed November 20, 2014.
13. Nelson C, Hojvat S, Johnson B, et al. Concerns regarding a new culture method for Borrelia burgdorferi not approved for the diagnosis of Lyme disease. Morbidity and Mortality Weekly Report 2014; 63:333.
14. Johnson BJ, Pilgard MA, Russell TM. Assessment of new culture method for detection of Borrelia species from serum of Lyme disease patients. Journal of Clinical Microbiology 2014;52:721–4.
15. Zhang X, Martin IM, Peña AC, Hopkins AB, Wroth L, Fix AD. Economic impact of Lyme disease. Emerging Infect Dis. 2006;12(4):653-660. Accessed April 16, 2015.
16. $1,226 = ($464 x 1.59 + $307 x 1.59).
17. U.S. Department of Labor, Bureau of Labor Statistics (BLS). CPI Databases. 2013. Accessed March 26, 2013.