Those who are sold false Chronic Lyme disease diagnoses (and frequently other unrecognized conditions) are being convinced to empty their wallets on expensive regimens of herbs and supplements.
Patients desperate for a solution to their problems sometimes crowdfund on sites like GoFundMe to fund these treatments.
A 2015 study reviewing “unorthodox” therapies marketed to treat Lyme disease—including herbs and supplements—concluded:
Providers of alternative therapies commonly target patients who believe they have Lyme disease. The efficacy of these unconventional treatments for Lyme disease is not supported by scientific evidence, and in many cases they are potentially harmful.
The CDC concurs:
Antibiotics are the only known effective treatment for Lyme disease, but a quick search on the internet will introduce you to other untested remedies that claim to cure Lyme disease or chronic Lyme disease. These products—available online or from some health care providers—may be dangerous, deadly, or simply a waste of money.
Table of contents
A pharmacist explains herbs & supplements
Scott Gavura, a pharmacist, gave an educational talk on the science of supplements. He then summarized the talk on Science-Based medicine. In this excerpt, Gavura explains:
A supplement worth taking is backed by good evidence, and is unlikely to cause harm. Most supplements fail this test – usually because they lack good evidence to show they work. But even where the evidence is promising (and the risks appear acceptable), there’s an additional consideration with dietary supplements – supplement quality is unclear.
When you consider the different products that are considered supplements, multivitamins and minerals are probably the safest product – the ingredients are known, they can be measured, and the manufacturing can be standardized. There’s more risk with the herbal and botanical remedies. Not only is there a lack of consistency, there’s a much greater risk of adulteration and contamination. Finally, sexual enhancement, weight loss, and body building supplements seem most likely to be adulterated (and there’s no reason at all to take these products).
When it comes to dietary supplements, there’s no easy way for a health professional or a consumer to independently verify that a product is of high quality. Regulatory structures leave a lot to be desired, resulting in a “buyer beware” marketplace for consumers. Until something changes, it will continue to be nearly impossible to have confidence in the quality of supplements, or to use them in science-based ways.
To learn more about the pharmacology of supplements, read the whole piece or watch the talk on “Where Science Meets Supplements”:
Herbs and Supplements: Bad laws hurt consumers
John Oliver on his show Last Week Tonight described how politicians in America passed laws like DSHEA that leave consumers vulnerable to false and misleading claims by the supplement industry:
Beware the “Quack Miranda Warning”
Herb and supplements will usually have a lengthy disclaimer similar to the following, because it is a requirement of the sham law called DSHEA:
“These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.”
Dr. Peter Lipson called this the “Quack Miranda Warning” because it reminded him of the Miranda warning given to suspects by law enforcement. Lipson opines:
There are three ways to look at this: the truthful way, the sinister way, and the bat-shit insane way.
- Truth: Anyone who wants to sell you something that’s a load of crap must use this statement to cover themselves legally.
- Sinister: Variation of above–someone wants to sell you something that you are supposed to believe is medically useful, but at the same time they tell you in fine print that it is not medically useful. When it doesn’t work, they don’t get sued. I wonder why anyone would buy something with that disclaimer attached to it? When I treat someone for a medical problem, I pretty much say that I intend to diagnose, treat, cure, or prevent a disease. Why would I say otherwise? It would be a lie. Also, who would go to see a doctor that told you that they didn’t intend to diagnose or treat disease. The whole thing is bizarre.
- Bat-shit insane: The FDA and Big Pharma are in cahoots with the AMA to keep you from learning all the simple ways to treat diseases. They want your money, and they’ll do anything they can to get it from you, including suppressing the knowledge than anyone can learn to heal cancer.
I can’t really help the people who believe #3, but people who are willing to suspend their paranoia should read #’s 1 and 2 a few times. Unless you’re being arrested, no one should be reading you your rights. The Quack Miranda Statement is the red flag that should send you running.
Weak evidence
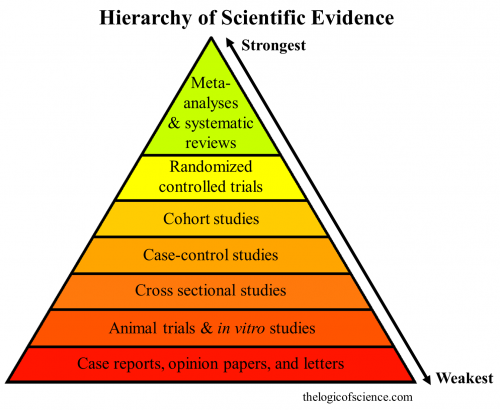
Different forms of evidence range in terms of quality. Most herbs and supplements do not have high quality evidence supporting their safety and efficacy.
Sometimes a headline might claim an herb or supplement kills Lyme disease, but closer inspection will show that evidence to support the claim is weak. Many studies are performed in petri dishes (in vitro) or in animals, not in humans.
Double-blind randomized controlled trials are the gold standard for determining the safety and efficacy of a treatment. In these studies, a randomly assigned group receives placebo and another group receives the experimental treatment. The effect of the treatment can be determined by comparing the two groups.
More detail on the different types of evidence can be found at The Logic of Science.
Not scientific evidence:
- YouTube and Netflix videos
- Personal anecdotes (“It worked for me!”)
- Gut feelings
- Parental instincts
- some guy you know
- Chronic Lyme support groups
- Lyme literate doctors
- Functional and integrative medicine practitioners
- Naturopaths, chiropractors, and homeopaths
- Internet surveys like MyLymeData
- Other red flags of Chronic Lyme quackery
Related: Why essential oils can’t treat Lyme disease
Anyone can be tricked
The influential physicist Richard Feynman famously said:
The first principle is that you must not fool yourself – and you are the easiest person to fool.
Some people invariably become convinced that an ineffective treatment works for them. But, as Dr. Harriet Hall describes in her course on Science-Based Medicine, we humans may believe ineffective treatments work for many reasons:
- The disease may have run its natural course.
- Many diseases are cyclical.
- We are all suggestible.
- The wrong treatment may get the credit.
- Diagnosis and prognosis may be wrong.
- Temporary mood improvement confused with cure
- Psychological needs affect behavior and perceptions
- We confuse correlation with causation
Dr. Barry Beyerstein, a professor of psychology, described the above reasons in his essay “Why Bogus Therapies Seem to Work”. He concluded:
To people who are unwell, any promise of a cure is especially beguiling. As a result, false hope easily supplants common sense. In this vulnerable state, the need for hard-nosed appraisal is all the more necessary, but so often we see instead an eagerness to abandon any remaining vestiges of skepticism.
Erstwhile savvy consumers, felled by disease, often insist upon less evidence to support the claims of alternative healers than they would previously have demanded from someone hawking a used car. Caveat emptor!
Unscientific treatments may leave real Lyme disease untreated
In almost all “Chronic Lyme” cases, Lyme infection was not the source of the patient’s problem in the first place. But if the patient did have Lyme disease or another tick-borne illness, pseudoscientific treatments like herbs and supplements likely wouldn’t cure the easily curable infection.
Lyme disease rarely remains untreated. However, an interesting 2011 report described a 71-year old woman who knew she had Lyme disease but refused antibiotic treatment. Instead, she chose acupuncture and homeopathy, which don’t work for any condition.
The woman had typical symptoms of Lyme disease, including the characteristic rash, which progressed to Lyme arthritis. Four years after her initial infection, the woman finally accepted antibiotic treatment and was quickly cured of the arthritis which had been bothering her. The effective treatment was per the science-based guidelines: 30 days of doxycycline.
Resources
LymeScience: Samento and Cat’s Claw
Mayo Clinic: Diagnosis and treatment of Lyme Disease (see “Alternative Medicine” section)
CDC: Alternative treatments for Lyme disease
Skeptical Inquirer: Why Bogus Therapies Seem to Work
Dr. Harriet Hall: Science-Based Medicine course
Lantos PM, et al. Unorthodox alternative therapies marketed to treat Lyme disease. Clin Infect Dis. 2015.
The Logic of Science: The hierarchy of evidence: Is the study’s design robust?
Dr. Steven Novella: Herbs are drugs
Dr. Jen Gunter: The Trouble with Turmeric
GID M-K: Essential oils can’t treat Lyme disease
Skepp: Herbal Remedies for Lyme?
Washington Post: Most dietary supplements don’t do anything. Why do we spend $35 billion a year on them?
Ars Technica: Supplements are a $30 billion racket—here’s what experts actually recommend
Navarro VJ, et al. Liver injury from herbal and dietary supplements. Hepatology. 2017.
Star Tribune/AP: Testing finds many herbal supplements don’t contain what the label says; fillers, not herbs
Thompson A, Hynicka LM, Shere-Wolfe KD. A Comprehensive Review of Herbal Supplements Used for Persistent Symptoms Attributed to Lyme Disease. Integr Med (Encinitas). 2023. [published in a quack journal]
Harms of herbs and supplements peddled for Lyme disease
LymeScience: Tainted Glutathione likely poisoned “Chronic Lyme” patients in Australia, where there is no Lyme disease [source]
Perrillo RP, et al. Herbal hepatitis due to use of alternative medicines for Lyme disease. Baylor University Medical Center Proceedings. 2021.
Flythe JE, et al. Silicate nephrolithiasis after ingestion of supplements containing silica dioxide. Am J Kidney Dis. 2009;54(1):127-30.
Patmas MA. Eosinophilia-myalgia syndrome not associated with L-tryptophan. N J Med. 1992.
Haden M, et al. Toxic levels of glycosides in herbal medication: a potential cause of hyperkalaemia. Scott Med J. 2011;56(4):236. [excerpts]
Tabbalat RR. Two Cases of Gastrointestinal Delusional Parasitosis Presenting as Folie á Deux. ACG Case Reports Journal. 2019.
Weiss E, et al. Side-by-side Comparison of a Picosecond 755-nm Alexandrite Laser and a Quality-switched 1064-nm Neodymium-doped Yttrium Aluminum Garnet Laser in the Treatment of Argyria. Cureus. 2019;11(7):e5206.
Newman M, Kolecki P. Argyria in the ED. Am J Emerg Med. 2001;19(6):525-6. [comment]
Natelson EA, et al. Anemia and leukopenia following intravenous colloidal silver infusions-Clinical and hematological features, unique peripheral blood film appearance and effective therapy with supplemental oral copper and apheresis. Clin Case Rep. 2019;7(9):1757-1762.
Philips CA, et al. Complementary and alternative medicines and liver disease. Hepatology Communications. 2024.
FDA, FTC, and other government actions
- American Chinese Medicine Association (Bob Xu)
- BioStar Technology International, LLC, 2017
- Bismacine, 2006
- Blue Ridge Silver, 2019
- Chronic Lyme Treatments (Sal Meli, Apex Botanicals, pdf)
- Desert Alchemist LLC, 2020
- East West Health, 2020
- Golden Sunrise Nutraceutical and Golden Sunrise Pharmaceutical (case, press release, Stephen Meis)
- Herbs of Light
- Hawaiian Organic Noni, LLC, 2018
- Herbal Vitality, Inc., 2023
- Humaworm, 2017, 2020
- KetoKerri LLC, 2020
- Kris A. Pletschke/Raw Health (order, letter, judgment)
- The LaCava Center, 2020 (Robert J. LaCava, MD and Suzanne LaCava, RN)
- Natural Alchemist, 2017
- New Earth Healing Essentials, 2022
- Organic Heirloom Plants, 2023
- PRL Inc, 2019
- R3 Stem Cell, LLC (letter, news release, David Greene)
- Results RNA: Recall, letters in 2018, 2019, 2021
- RichSource Stem Cells, 2019
- Robert Spencer, Lisa Spencer, Aaron Company (complaint, exhibit, analysis, consent)
- Seasilver, 2003
- South Texas Botanicals, 2018
- Viviane Schaller, Bernard Christophe, and Tic Tox (articles)
- Westberry Enterprises and Timothy Westberry (complaint)
- Wholly Liquid Nutritional Supplements LLC (Douglas A. Wine, Austin A. Wine)
Other regulatory actions
Park Avenue Stem Cell and Joel B. Singer, complaint
updated October 4, 2024