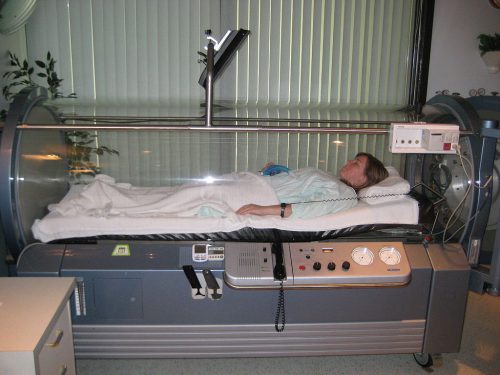
Hyperbaric Oxygen (HBOT) is another unnecessary therapy marketed for Lyme disease.
The FDA and Inside Edition have warned the public about cure-all claims made about HBOT. According to the FDA:
HBOT has not, however, been proven to be the kind of universal treatment it has been touted to be on some Internet sites. FDA is concerned that some claims made by treatment centers using HBOT may give consumers a wrong impression that could ultimately endanger their health.
Another statement by the FDA specifically cautions regarding unproven Lyme disease treatment:
If you are considering the use of a HBOT device for yourself or a loved one, be aware that some claims of what it can do are unproven. For example, HBOT devices are not proven to cure cancer, Lyme disease, autism or Alzheimer’s disease. The U.S. Food and Drug Administration recommends you check with your health care provider before using a HBOT device to make sure you are pursuing the most appropriate care.
A 2015 review of 30 unproven or disproven therapies advertised for Lyme disease noted “No study of [HBOT] in humans with Lyme disease has ever been published.” A 2025 study found 117 US clinics that sold “nontraditional” (read: quack) Lyme disease treatments, and 15% of the clinics sold HBOT.
The Associated Press and Canadian Broadcasting Corporation have reported on the industry that profits from fake Lyme tests and treatments. This industry is assisted by misinformation and conspiracy theories, which help breed distrust of mainstream medicine.
HBOT is not cleared as safe and effective for Lyme disease by the FDA, and HBOT is not approved for Lyme disease by the Undersea and Hyperbaric Medical Society (UHMS).
Hyperbaric oxygen failed against Lyme
In a 2018 conference presentation, staff at Dartmouth Hitchcock Medical Center reported on a case of a 77-year-old man who was being treated with HBOT for radiation cystitis. The man had a tick bite, had 8 HBOT sessions, and still developed the classic erythema migrans (EM) rash of Lyme disease (LD). The report concluded:
Although this is a single case, the fact that the patient developed LD during HBOT does not support the hypothesis that HBOT prevents LD.
Hyperbaric oxygen side effects
Johns Hopkins lists a series of side effects that can occur from HBOT inside a hyperbaric chamber. Though most side effects are generally mild, HBOT is not risk-free.
In 2025, 5-year-old Thomas Cooper was killed by an explosion in a hyperbaric chamber. Dr. David Gorski noted:
It was soon reported that at the time of his death, Thomas had been undergoing HBOT for indications that are most definitely not evidence- or science-based, specifically ADHD and sleep apnea. In other words, he was the victim of quackery and an unscrupulous quack.
Charlatans abound
- Former ILADS president Samuel Shor, MD was found to have committed unprofessional conduct by horrifically mistreating a patient. He has a history of selling HBOT for Lyme disease.
- Former ILADS board member Phillip DeMio, MD had his license revoked for mistreatment of 5 adults and 11 children. The revocation documents DeMio’s quack claims of being able to treat autism, Lyme disease, and other disorders with HBOT.
- The license of Kenneth Paul Stoller, MD was revoked for endangering children with bogus vaccine exemptions. Previously, he had dodged accusations connected to the Medical Child Abuse of a young girl who he treated with HBOT.
- Giuseppina Benincasa-Feingold, MD is another hyperbaric oxygen seller who has a history of misconduct. Benincasa-Feingold’s site claims that HBOT can treat over 40 conditions, including Lyme disease. Benincasa-Feingold was a speaker at conference organized by the abhorrent Lyme-induced Autism Foundation. Lyme disease does not cause autism.
- Nurse Julia Sudylo peddled hyperbaric oxygen and was disciplined for the unauthorized practice of medicine.
Sources:
- FDA: Hyperbaric Oxygen Therapy: Get the Facts
- FDA: Hyperbaric Oxygen Therapy: Don’t Be Misled (archived)
- CDC: Alternative treatments for Lyme disease
- Government of Canada: Hyperbaric Oxygen Therapy
- Johns Hopkins: Complications of Hyperbaric Oxygen Treatment
- Unorthodox Alternative Therapies Marketed to Treat Lyme Disease, 2015
- Inside Edition: Miracle Hyperbaric Treatment Claims Prey On Those Desperate For A Cure, 2014-11-06
- Dr. Christopher Labos: Hyperbaric oxygen therapy may just be hot air for many, 2024-09-24
- CMAJ: Unregulated hyperbaric oxygen therapy clinics assailed, 2010
- Ptak J, Reetz S, Buckey J. Lyme Disease Developing During Hyperbaric Oxygen Treatments. Annual meeting of The Undersea and Hyperbaric Medical Society. 2018.
- Sakizadeh JR, Rothenberger MK, Alpern JD. Characteristics of Clinics Offering Nontraditional Lyme Disease Therapies in Lyme Endemic States of the United States. Open Forum Infect Dis. 2025
- Dr. David Gorski: Quackery (still) kills: A five-year-old boy dies in a hyperbaric oxygen chamber, 2025-03-17
- NBC News: Hyperbaric chamber facility where boy died put profits before client care, Michigan attorney general says, 2025-03-11
- Associated Press: Unproven Lyme disease tests and treatments are proliferating, 2025-09-10
Updated January 27, 2026