LymeScience republishes:
Anchoring Bias on Lyme Disease Leading to Delayed Diagnoses
- Captain Lacy Lowry, MD, US Air Force Medical Corps, Department of Internal Medicine, Brooke Army Medical Center
- Major Joseph Yabes, MD, US Air Force Medical Corps, Infectious Disease Service, Department of Medicine, Brooke Army Medical Center
- Major Heather Pomerantz, MD, US Army Medical Corps, Infectious Disease Service, Eisenhower Army Medical Center
- Lieutenant Colonel Alice E. Barsoumian, MD, US Air Force Medical Corps, Infectious Disease Service, Department of Medicine, Brooke Army Medical Center
To the editor—Lyme disease was first described in Lyme, Connecticut in 1976, with the discovery of the causative organism several years later. Since 1995, the reported cases of the illness have tripled, with the Centers for Disease Control and Prevention now estimating 300,000 new cases annually in the United States.
The Military Health System is likewise affected, with Lyme disease being the most commonly diagnosed vector-borne disease. However, diagnosis of Lyme disease can be challenging, requiring a combination of epidemiologic risk factors, clinical symptoms, and laboratory testing. Therefore it is unsurprising that misdiagnosis of Lyme disease and complications from misdiagnosis have also increased.
We explore 3 cases of delayed diagnosis because of initial misdiagnosis of Lyme disease, and discuss how cognitive biases can affect clinical reasoning and patient outcomes.
Case 1
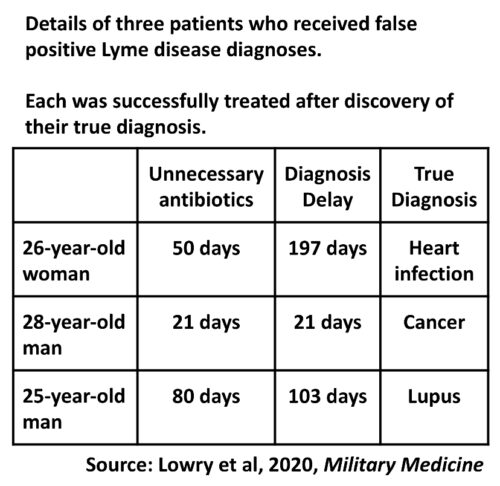
A 26-year-old woman presented with a 1 year history of polyarthritis, night sweats, fatigue, and a 17 pound weight loss. After several months of illness, a diagnosis of Lyme disease was made based upon a positive enzyme-linked immunoassay (EIA) and immunoglobulin M (IgM) results on western blot. She completed multiple courses of antibiotics without improvement before eventual referral to the clinic (Table I).
TABLE I: Laboratory Results and Clinical Features of Cases Misdiagnosed as Lyme Disease
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Exposure risk | None | Resided in Lyme-endemic area 4 years before symptom onset | None |
| Lyme test results | |||
| EIA | Positive | Positive | Positive |
| IgM | Positive | Positive | Negative |
| IgG | Negative | Negative | Negative |
| Interpretation in clinical context | Negative | False-positive | Negative |
| Rationale | > 30 days of symptoms | No recent exposure | Negative confirmatory testing |
| Lyme-directed treatment(s) | Doxycycline, 10 days Doxycycline, 30 days Amoxicillin, 10 days | Doxycycline, 21 days | Doxycycline, 21 days Amoxicillin, 14 days Doxycycline, 45 days |
| Final diagnosis | Subacute infective endocarditis | Papillary thyroid cancer | Systemic lupus erythematosus |
| Duration of delay in diagnosisa | 197 days | 21 days | 103 days |
aFrom the date of Lyme misdiagnosis to the date of final diagnosis.
Physical examination revealed a grade III/VI systolic murmur. Cardiobacterium hominis grew from blood cultures and she was hospitalized for expedited evaluation. The patient was diagnosed with mitral valve infective endocarditis based on echocardiogram and clinical features.
Her clinical course was complicated by the onset of a new headache and left visual field deficit. Right occipital lobe septic embolism and mycotic aneurysm were diagnosed by computed tomography angiography. She underwent coil embolization, completed 6 weeks of antibiotics, and required a mitral valve repair, after which her symptoms resolved.
Case 2
A 28-year-old male presented for evaluation of submandibular lymphadenopathy. The patient discovered a new submandibular mass 2 weeks before presentation. Lyme serologies were ordered based on remote residence in a Lyme-endemic area. The patient had a positive EIA and IgM western blot, prompting doxycycline initiation (Table I) and referral to infectious disease.
Physical examination showed submandibular lymphadenopathy but was otherwise unremarkable. Review of systems revealed a 2-year history of drenching night sweats. Histopathology of an excisional biopsy revealed papillary thyroid cancer. Thyroidectomy was performed, with symptom resolution thereafter.
Case 3
A 25-year-old male presented with 5-month history of chills, night sweats, and polyarthralgias. The pain was worse in the morning, and improved with nonsteroidal anti-inflammatories. Despite negative western blot confirmatory testing, he was diagnosed with Lyme disease and prescribed antibiotics (Table I). His symptoms persisted despite repeat courses of antibiotics, and he was referred for further management.
Physical examination displayed reduced active range of motion on flexion of the shoulders and hips bilaterally and inflammatory arthritis was suspected. Rheumatologic workup revealed an elevated antidouble-stranded DNA and PM/Scl-100 antibodies. Magnetic resonance imaging of the upper extremities displayed tenosynovitis and partial tendon tears. The patient was diagnosed with systemic lupus erythematosus. Hydroxychloroquine, leflunomide, and prednisone were initiated, with symptomatic improvement.
Discussion
Lyme disease, caused by Borrelia burgdorferi, is the most common tick-borne infection in the United States. The diagnosis of Lyme disease is of particular relevance within the Military Health System.
Multiple military bases are located within Lyme-endemic areas of the country. Therefore even service members presenting with the appropriate clinical syndrome and stationed in nonendemic regions should receive a thorough travel history as they might have previously been stationed in areas at risk. Furthermore, field training aimed at promoting readiness may also serve to increase the risk of disease acquisition.
Accurate diagnosis requires assessment of geographic and exposure risk factors as well as symptom duration during the initial interview. In the absence of erythema migrans, diagnosis requires a two-tier laboratory algorithm, consisting of an initial EIA screening assay, followed by interpretation of a IgM and/or immunoglobulin G (IgG) western blot confirmatory test based upon symptom duration.
A negative IgG excludes Lyme disease for symptoms present for more than 30 days. Misinterpretation of these tests decreases their specificity, leading to higher false-positive rates. Such results, in conjunction with anchoring bias, led to these cases of misdiagnosis.
Of note, since the time of initial patient evaluation the Centers for Disease Control and Prevention has updated their diagnostic guidelines to recommend a second EIA serologic assay as an alternative to western blot.
Anchoring bias is a cognitive bias that refers to maintaining a diagnosis in spite of contradictory information.
In Case 1, the patient was diagnosed with Lyme disease based upon an incorrect risk assessment and misinterpretation of laboratory results. Despite ongoing symptoms after treatment, alternate diagnoses were unexplored.
In Case 2, the patient was diagnosed based on IgM banding performed in a patient with no geographic risk factors for early Lyme disease.
Finally, in Case 3, the patient was diagnosed despite no risk factors, negative testing, and lack of symptomatic improvement with prolonged antibiotic therapy.
Although the providers had inexperience with accurate risk assessment and test interpretation, the cases were complicated by the failure to reexamine the diagnosis after poor clinical response.
We encourage providers to be aware of potential cognitive biases to maximize clinical reasoning and lead to better patient care. When initial management is unsuccessful, reexamination of clinical cases, presenting symptoms, and laboratory data may be a useful strategy to guard against anchoring bias.
References available from the authors.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, or the Department of Defense or the U.S. Government.
Originally published in Military Medicine [pdf] by Oxford University Press on behalf of the Association of Military Surgeons of the United States 2020. LymeScience fixed typos and reformatted for optimal web browser display.
Citation: Lowry L, Yabes J, Pomerantz H, Barsoumian AE. Anchoring Bias on Lyme Disease Leading to Delayed Diagnoses. Mil Med. 2020;usaa130.
This article is a U.S. Government work and is in the public domain in the USA.

More resources:
- Jonathan Howard, MD: Neurologist explains chronic Lyme false assumptions
- LymeScience: Lyme Misdiagnosis Illustrated
- Webber BJ, et al. Lyme Disease Overdiagnosis in a Large Healthcare System: A Population-based, Retrospective Study. Clin Microbiol Infect. 2019.
- Nelson RE, et al. Evaluation of serological testing for Lyme disease in Military Health System beneficiaries in Germany, 2013-2017. MSMR. 2019;26(8):22-26. (HTML | PDF)