LymeScience republishes:
Lyme disease: “End of the debate?”
Guillaume Coiffier a,c,d*, Pierre Tattevin b,c.
a Service de Rhumatologie, GHT Rance-Émeraude, CH Dinan, 74 boulevard Chateaubriand, 22100 Dinan, France
b Service de Maladies Infectieuses & Réanimation Médicale, Pontchaillou, CHU Rennes, 33 boulevard Louis Guilloux, 35000 Rennes, France
c Centre de Référence des Maladies Vectorielles à Tiques (MVT), Pontchaillou, CHU Rennes, 33 boulevard Louis Guilloux, 35000, Rennes, France
d Groupe de travail sur les Infections Ostéo-articulaires, Société Française de Rhumatologie (SFR), Paris, France
* Corresponding author: Dr. Guillaume Coiffier, Service de Rhumatologie, GHT Rance-Émeraude, CH Dinan, 74 boulevard Chateaubriand, 22100 Dinan, France. ORCID ID 0000-0003-3560-128X.
PDF manuscript | HAL | Published paper
Abstract
Lyme borreliosis is a tick-borne disease that is widespread throughout the northern hemisphere. Ixodes ricinus is present throughout metropolitan France, except for the Mediterranean region. The debate revolves around whether or not a chronic form of Lyme disease exists. This controversy is not limited to France but has been reported worldwide.
In France, in 2019, 24 scientific societies representing the medical disciplines most involved in Lyme disease, including the Société Française de Rhumatologie (French Rheumatology Society – SFR) and the Société de Pathologie Infectieuse de la Langue Française (French Infectious Disease Society – SPILF), published recommendations on the management of Lyme borreliosis following a submission to the Director General of Health. These recommendations conflict with those of the Haute Autorité de Santé (HAS), a multi-specialties independent group of physician, on a key point: whether to add a new nosological entity labeled as “persistent polymorphous signs and symptoms (or syndrome) possibly due to tick bite.” The creation of this new syndrome risks should increase anchoring bias, leading to the attribution of all symptoms to a possible tick bite, without considering differential diagnoses.
Lyme disease has been extensively studied. Erythema migrans is the primary clinical manifestation. In the presence of nonmetabolic, nonseptic monoarthritis involving the knee or radiculitis of a lower limb during the summer, Lyme disease should be suspected. Serologic testing for Lyme disease is reliable in the case of late forms such as chronic arthritis, while the detection of Borrelia DNA in synovial fluid by PCR is inconsistent. Sometimes, the serology can be misleading in early forms such as radiculitis. Treatment is based on doxycycline for 14 days in early forms (radiculitis), or 28 days in late forms (arthritis). Arthritis can persist or recur after antibiotic therapy.
The prevalence of a diffuse polyalgia syndrome (fibromyalgia) following Lyme disease does not seem to differ much from that in the general population. It is not improved by prolonged antibiotic therapy, which is therefore not recommended.
Highlights
- Lyme disease is a vector-borne infectious disease (Borreliella) transmitted by ticks (Ixodes ricinus) present in metropolitan France.
- The primary clinical manifestation of Lyme disease is erythema migrans.
- In the presence of nonmetabolic, nonseptic monoarthritis of the knee, or summertime radiculitis of a lower limb, rheumatologists should suspect Lyme disease.
- Lyme disease serology is reliable in the case of arthritis, while Borrelia DNA detection in synovial fluid by PCR is inconsistent (40%).
Key words: Lyme disease, arthritis, radiculitis, erythema migrans, recommendations
1. Introduction
Lyme disease is a vector-borne disease or metazoonosis: a Borreliosis transmitted to humans by a mite of the genus Ixodes sp. (hard ticks of different species in various parts of the world), which in turn carries a pathogenic spirochete acquired during a previous blood meal, usually from small birds or mammals. The primary clinical manifestation of this disease is erythema migrans (EM), a rash that expands outward from the inoculation point (Figure 1). Secondary cutaneous, joint, neurological, cardiac, or even ophthalmologic manifestations may be observed [1].
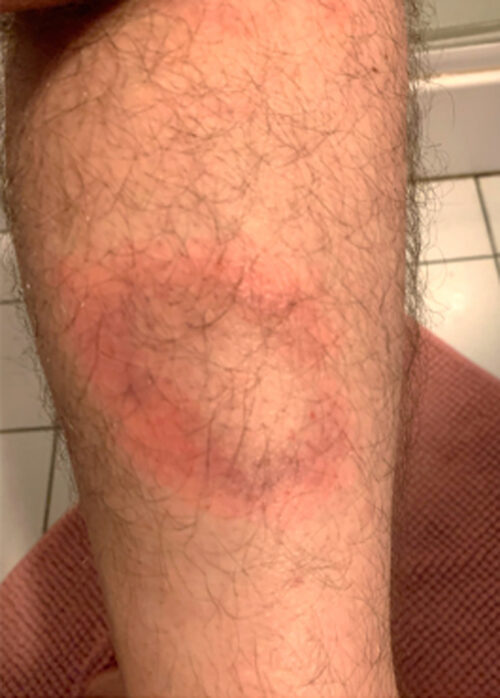
(Personal collection of P. TATTEVIN)
The earliest evidence of human borreliosis is very old. A genomic study of Ötzi, a mummified cadaver dating back 5,300 years, found traces of bacterial DNA from Borrelia burgdorferi [2], identified in 1982 as the infectious agent that causes Lyme disease [3]. The first descriptions of EM date back to the early 20th century, although there was no known link to the pathogen or vector. The disease is named for the city of Lyme, Connecticut, in the United States, where an epidemic of EM and joint inflammation occurred in 1975 [4]. The vector was identified in 1978 and the bacteria in 1982 [3,4].
1.1 This disease seems to have been extensively studied… so what is the matter of controversy?
The debate revolves around whether or not a chronic form of Lyme disease exists [5]. In France, in 2018, the debate crystallized between the Haute Autorité de Santé (HAS)1 and 12 learned societies, representing the medical disciplines most concerned with Lyme disease, including the Société Française de Rhumatologie (French Rheumatology Society – SFR) and the Société de Pathologie Infectieuse de la Langue Française (French Infectious Disease Society – SPILF), which refused to sign on to HAS’s proposed recommendations on Lyme disease. The main reason for the disagreement was the mention of a new entity called “persistent polymorphous signs and symptoms (or syndrome) possibly due to tick bite (SPPT),” which was not supported by the pathophysiological evidence, posing a risk of overdiagnosis that could lead to prolonged and inappropriate antibiotic therapy.
In 2015, the Fédération française des maladies vectorielles à tiques (French federation of tick borne disease – FFMVT), a coalition of four associations—France Lyme, Lyme sans Frontières, Lympact, and Relais de Lyme—began pleading for official recognition of chronic Lyme disease and became “a credible spokesperson for regulators and health authorities,” allowing the SPPT entity to be entered in the good practice guide of HAS [6].
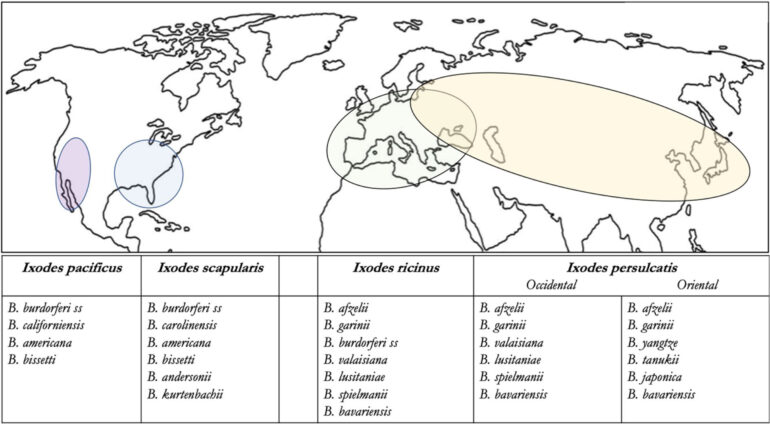
Their arguments were based on several ideas. Borrelia burgdorferi is an “invisible” bacterium because of how hard it is to culture (low bacterial inoculum in Lyme disease and requires a special growth medium). The diagnosis is usually made indirectly by serology. The large number of manufacturers can make diagnostic performance variable and imperfect, as with any serologic test. In addition, performance depends on the disease stage, with false negatives possible in the first weeks after infection. For detractors of these tests, this is enough to arouse mistrust. Ixodes ricinus can also transmit pathogens other than those that cause Lyme disease (Babesia microti, Francisella tularensis, Anaplasma sp., Ehrlichia sp., Coxiella burnetii, tick-borne encephalitis virus), and certain doctors associated with the FFMVT (“Lyme doctors”) believe that these co-infections could be partly responsible for other chronic symptoms expressed by patients, with no convincing scientific proof. Moreover, the number of identified subspecies of Borrelia sp. [7] infesting ticks of the genus Ixodes sp. is constantly increasing (figure 1, table 1), which feeds fantasies about their disease involvement and the reliability of diagnostic testing, despite a poorly understood pathogenesis. The confusion entertained, consciously or not, between Lyme disease and tick-borne disease promotes doubt about test reliability and belief in a possible chronic form of Lyme disease (in the sense of a tick-borne disease) with a negative serology.
Table 1. Taxonomy of Lyme disease spirochetes and pathogens
| Order | Spirochaetales | |||
| Family | Borreliaceae | Spirochaetaceae | Leptospiraceae | |
| Genus | Borreliella | Borrelia | Treponema | Leptospira |
| Species | B. burgdorferi ss B. afzelii B. garinii B. bavariensis B. spielmanii B. lusitaniae B. mayonii B. valaisiana B. bissettii B. carolinensis B. americana B. andersonii B. californiensis B. finlandensis B. kurtenbachii B. japonica B. tanukii B. turdi B. sinica B. yangtzensis B. chilensis | B. miyamotoi B. recurrentis B. duttoni B. hermsii | T. pallidum T. endemicum T. pertenue T. carateum | L. interrogans L. icterohaemorrhagiae, L. canicola, L. autumnalis, L. australis, L. grippotyphosa L. pyrogenes L. weilii, L. santarosai, L. noguchii, L. borgpetersenii, L. kirschneri L. biflexa L. inadai L. meyeri |
| Human diseases | Lyme disease | Recurrent fevers | Syphilis Pian, Bejel, Pinta | Leptospirosis |
Bold: pathogen implicated in human Lyme disease (EM at a minimum); Gray: Borrelia genetically related to Borreliella found in hard ticks of the genus Ixodes, potentially pathogenic in animals with no known involvement in human Lyme disease
Finally, symptoms of chronic fatigue and diffuse musculoskeletal pain may occur temporarily after infection (infectious mononucleosis, chronic brucellosis, chikungunya, etc.) and can thus suggest a similar phenomenon in Lyme disease. Some animal models suggest that Borrelia burgdorferi could have the ability to encyst itself, remaining in a dormant and persistent state in the body [8]. Even though it involves preliminary data that are not yet confirmed in humans, these experimental data give fuel to the Lyme doctors’ arguments in favor of prolonged, combined antibiotic therapies, even though high-level randomized controlled studies have found these strategies to have no benefit.
It must be remembered that chronic fatigue syndromes and fibromyalgia are common in today’s societies and there are many other contributing factors. As with all functional symptoms, a familiar cascade of fears and beliefs can take root: the symptom echoes a negative test result and therefore absence of a “disease” diagnosed by the doctor, which the patient immediately perceives as a lack of understanding (“I’m suffering,” “the test is negative,” “the doctor doesn’t believe me”) [9,10]. Feeling “misunderstood” by the doctor, the patient then turns to other sources of “medical” information, that is, the Internet! [6,11]. There we find another sort of literature, consisting of the testimonies of people who are suffering (like the patient) and who have not been immediately diagnosed (like the patient). The doctor’s negative test becomes “positive” when done by a private laboratory using unvalidated techniques. This time, the source of information is not scientific (evidence-based) but “political” in the lobbyist sense (based on an ideology shared by a certain number of people) and largely propagated by the FFMVT. This “political” current organizes and spreads selective information. As for COVID-19, the patient undergoes the devastating effects of media jousts between “experts,” “scientific journalists,” and “politicians.”
This French example is representative of a much more widespread situation in the world. All over the world, the clinical manifestations of Lyme disease and the questions surrounding it are the same. In the United States, the Infectious Diseases Society of America (IDSA), the American College of Rheumatology (ACR), and the American Academy of Neurology (AAN) just published joint recommendations in 2020 [12]. An IDSA public forum warns about the risk of delayed diagnosis and specific management of other pathologies when Lyme disease is “overdiagnosed.” Furthermore, this forum reminds us of the importance of research in providing answers to questions about this disease. In its absence, the scientific vacuum is filled by “inappropriate and potentially dangerous experimental treatments.” [13]
1.2 So, who to believe?
Human mind rejects unfairness (but disease is not “fair”) and seeks to assign blame. It is sometimes easier to point to a convenient culprit (such as a tick), rather than to long and complex interactions between the patient and the environment. This update aims to retrace the major stages of Lyme disease with particular attention on rheumatological symptoms and to explain the latest recommendations cosigned by French learned societies in 2019 [14,15], shared by most other societies around the world [13,16].
2. A change in taxonomy: Lyme disease is a Borreliella infection.
The pathogen responsible for Lyme disease is a bacterium of the spirochete class (which includes the family Borreliaceae but also Treponema pallidum, responsible for syphilis, and Leptospira sp. responsible for Leptospirosis) (Table 1).
In 1982, an American entomologist, Willy Burgdorfer, isolated the spirochete in the digestive tract of a tick (Ixodes scapularis) and demonstrated its connection to EM in humans: it was named Borrelia burgdorferi in his honor [3]. A short time later, other species associated with Lyme disease were described in Europe and Asia (B. afzelii, B garinii, B. spielmanii) that differed from those described a few years earlier in the United States, remained B. burgdorferi sensu stricto, while the group of Borrelioses implicated in Lyme disease were named B. burgdorferi sensu lato. In 1997, the genome of B. burgdorferi sensu stricto was sequenced [17]. Then, a large number of Borrelioses were discovered, leading to a suggestion in 2014 [18] that the Borreliaceae family be divided into two groups: the Borreliella group, combining all of the species capable of inducing EM (more than 20 species) (Figure 2), and the Borrelia group capable of inducing recurrent fevers (Table 1).
3. Epidemiology of Lyme disease: hidden epidemic of an emerging disease?
3.1. A hidden epidemic?
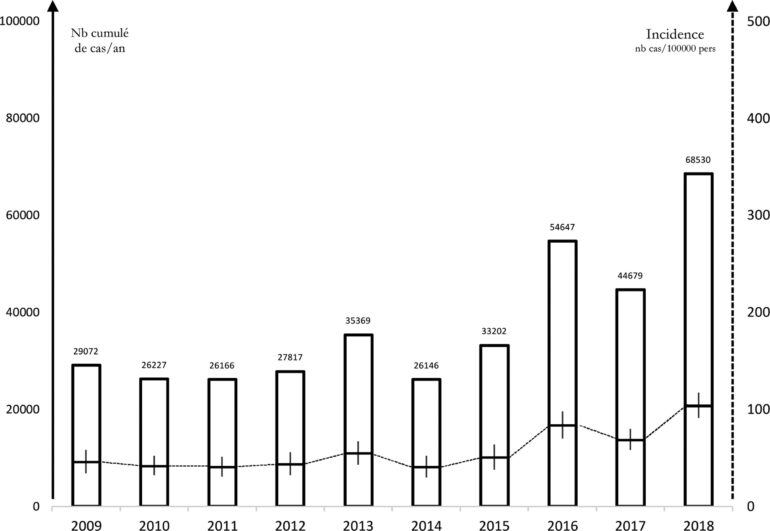
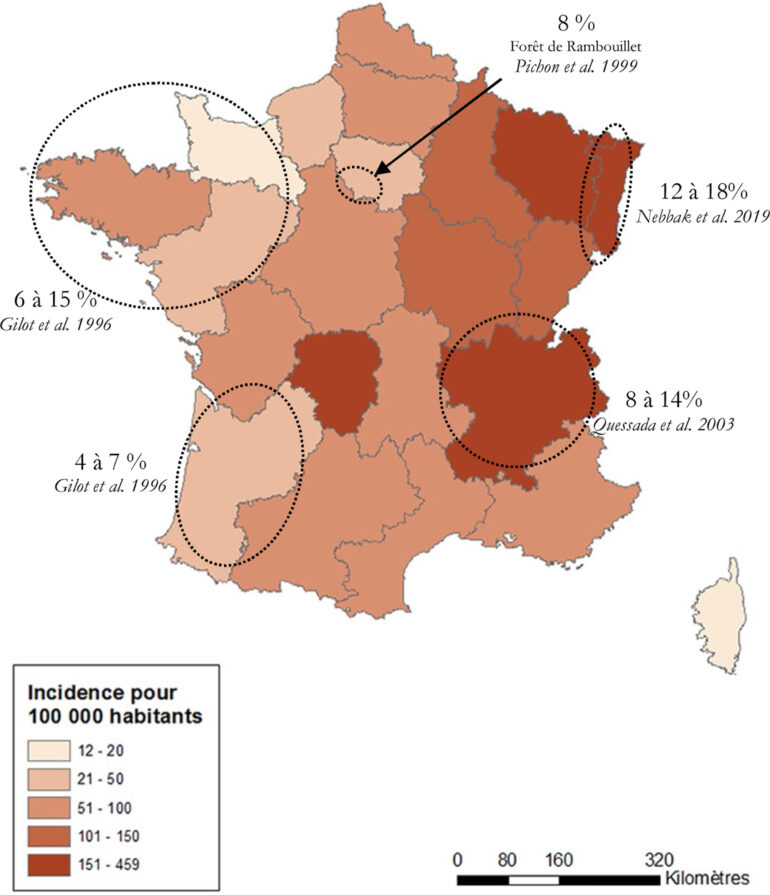
In France, epidemiological surveillance relies mainly on a sentinel network of primary care physicians, since Lyme disease leads to few hospitalizations or deaths. Between 2009 and 2017, this network reported an incidence of Lyme disease of approximately 50/100,000 people per year with a peak of 104/100,000 observed in 2018 [14] (Figure 3). The most common manifestation observed by the sentinel network was EM (95%). The incidence of hospitalization for Lyme disease is low, fewer than 1.5/100,000 people per year, mostly pediatric and neuroborreliosis cases [19]. The incidence in bordering countries (Germany, Belgium, and Switzerland) is the same. However, seroprevalence in the general population is thought to be higher, increasing with age and varying from one region to another (up to 20% in Alsace, for example) (Figure 4).
3.2 Is Lyme disease underestimated then?
A randomized, placebo-controlled study of the impact of Lyme disease prevention by the topical application of azithromycin after a tick bite provides some answers to this question [20]. The study evaluated 1371 patients with recent tick bites (< 72 hours) in an area where Lyme disease is endemic (Germany and Austria). Of these patients, 376 (27%) were excluded because they had a positive Lyme serology, making it impossible to interpret the main study criterion (i.e., the observation of EM or seroconversion). The infestation rate of Ixodes ricinus with Borreliella was 17%. No difference was shown between the two groups with regard to the main study criterion, with the appearance of 14 cases of EM (1.4%), of which 10 (5.7%) had proven infestation of ticks with Borreliella, with 12 (1.2 %) seroconversions at 2 months. This study supports an older American study of 482 people bitten by an Ixodes scapularis tick: 9/482 (2.3%) developed an EM following the disease (1 in the single-dose doxycycline group and 8 in the placebo group) [21]. Based on the results of these studies, starting from the premise that 100,000 people were bitten by Ixodes sp. in a region where the Borreliella infestation rate is 17% with a 5% risk of EM (a conservative scenario in a highly endemic area such as Austria), the incidence of EM can be estimated at 100,000 x 0.17 x 0.05, i.e., 850/100,000 patients. In France, the available data showed an infestation rate of Ixodes ricinus with Borreliella that varied from 4% in the Southwest to 18% in Alsace, depending on the region [22-25]. The incidence of Lyme disease is therefore probably “reasonably” underestimated, without increasing the epidemiological severity indices (rates of hospitalization and/or mortality).
3.3. An emerging disease?
The World Health Organization (WHO) defines an emerging infection as a new infection that reappears, or one for which the incidence has increased over the past two years, or that risks increasing in the near future [26]. Several factors are conducive to the emergence of infectious diseases: the properties of the infectious agent (virulence, resistance to anti-infectious agents, abilities to mutate and adapt to animal reservoirs), climatic and environmental factors favorable to the transmission vectors, the characteristics of the host/individuals (individual immunocompetence) and collective factors (demographics, societal behavior, and changes in the ecosystem).
If we consider all these points with regard to Lyme disease, it seems unlikely to be an emerging pandemic. In fact, Lyme disease is an “old disease,” with no interhuman transmission described, highly dependent on the environmental ecology, for which climate change and deforestation are not propitious to global expansion of the vector (Ixodes sp.) and the animal reservoir.
Conversely, certain endemic regions may emerge locally, and changes in human behavior may increase the risk of encountering ticks (enthusiasm for gardening, walks, and outdoor sports). These parameters require closer surveillance and preventive measures (rules for protecting against tick bites, self-observation to remove the tick quickly, etc.)
4. Main clinical manifestations found in rheumatology.
In France, Lyme disease has typically been divided into 3 progressive phases (primary, secondary, and tertiary), as with the stages described in syphilis, the other main infection with spirochetes [27]. This classification is outdated because it is a source of confusion between the tertiary phase (implying a chronic form of Lyme disease) and post-Lyme syndrome, described below. The clinical manifestations should be classified as early (< 6 months) or late (≥ 6 months) forms based on the time from the onset of symptoms from the date of inoculation with Borreliella. The earliest (and often only) symptom of Lyme disease is EM (Figure 1): a painless spot ranging from pale pink to dark red, surrounding the tick bite that appears 3 to 30 days after the bite occurred. As it progresses, erythema expands outward from the center leaving a “central clearing” of healthy skin, but not always. This acute, nonpainful form is rarely found in rheumatology. Serology is not necessary for diagnosis at the EM stage [14].
4.1. Lyme arthritis
Lyme arthritis may be observed after infection with all Borreliella species but more often after B. burgdorferi sensu stricto [28-32]. Its presentation in adults is relatively characteristic. It involves nonfebrile arthritis in a single knee (> 95%) that maybe either acute or chronic, is often recurrent, fluctuates greatly, with significant joint effusion, and is often associated with a popliteal cyst, of which rupture is not uncommon [28-32]. There is less pain than with common types of septic arthritis, and it is generally possible to walk. Oligoarticular forms that occur predominantly in the lower extremities occur exclusively in pediatric forms [33,34].
Arthritis occurs an average of 6 months after the tick bite (between 2 months and 2 years). Tick bites and EM, painless events, are rarely mentioned on the history intake (< 25% of cases). This manifestation occurs late and therefore IgG serology is always positive [28-32]. PCR testing may be requested to detect Borrelia sp. DNA in synovial fluid, but it is positive in only about 40% of cases (confirmed case). A synovium biopsy does not seem to improve PCR positivity compared with synovial fluid [33,34]. A negative PCR therefore does not rule out the possibility of Lyme arthritis, which must be treated if the serology is positive (probable case). On the other hand, a negative serology or a serology that is positive only on IgM does rule out Lyme arthritis.
After appropriate antibiotic therapy (doxycycline or ceftriaxone for 28 days), arthritis was cured in 70% to 90% of cases [28-34].
In about 10% to 30% of cases, persistent or recurrent arthritis was described after appropriate antibiotic therapy, called antibiotic-refractory Lyme arthritis (ARLA) [26-36]. This rare disease remains poorly understood but is thought to be more common after a B. burgdorferi sensu stricto infection. It is not a chronic or persistent infection, but rather a postinfectious immunological reaction mechanism [29,37,38]. The disease naturally runs its course within a maximum of 4 to 5 years, with or without immunomodulator treatments [31].
4.2. Lyme radiculitis
“Lyme radiculitis” is another common symptom that should cause the disease to be suspected. Bannwarth syndrome, first described in 1942, combines meningoradiculitis, predominantly in the lower limbs (often sciatic), “multiradicular” (extension of pain beyond dermatomes), insomnia, accompanied by headaches (common but aspecific), and facial paralysis (about 30% of cases, especially in children) [14,39]. This syndrome, which is usually incomplete, is suggestive of Lyme neuroborreliosis.
This is an early neurological manifestation that occurs during the EM stage or in the month following it, mainly in the summer or early autumn, the peak incidence in metropolitan France due to increased exposure during the summer months. The Lyme serology may be negative at this still early stage, particularly on the IgG test, and must be repeated 2 to 4 weeks later to confirm the diagnosis. A lumbar puncture is needed to detect lymphocytic meningitis associated with intrathecal synthesis (Lyme serology on CSF, measuring an indicator that documents intrathecal synthesis). The PCR test for Borrelia DNA in CSF is of little benefit because it is usually negative. A spinal MRI may show increased signal intensity at the root involved, and it is also advisable to rule out a differential diagnosis (disc or osteophytic root impingement) [40].
4.3. Acrodermatitis chronica atrophicans
Acrodermatitis chronic atrophicans (ACA) is not a musculoskeletal manifestation but it may be confused with a complex regional pain syndrome (formally called algodystrophy). This is a late cutaneous manifestation of Lyme disease, mainly with B. afzelii, affecting adults over 50 years of age. ACA is characterized by a (asymmetrical) dark red to violet-blue patch on part of a limb, most visible where the skin is in contact with the bone. It is edematous, progressing toward atrophy/sclerosis, often with significant allodynia. Lyme serology is consistently strongly positive on IgG. The skin biopsy shows collagen remodeling with telangiectasis and plasmocytic infiltrate and inconsistent positivity on the Lyme PCR [14].
4.4. Diffuse polyalgia syndrome (fibromyalgia) and Lyme disease: what do we know?
The IDSA defines post-Lyme syndrome based on a combination of 3 mandatory major criteria: 1) confirmed B. burgdorferi infection (EM and/or arthritis or neurological manifestation with positive serology), 2) improvement of these manifestations with appropriate antibiotic therapy, 3) then the onset of chronic, disabling subjective symptoms (≥ 6 months) within the 6 months following a confirmed diagnosis of B. burgdorferi infection. [41].
Diffuse musculoskeletal pain is thought to represent 15% to 30% of these “disabling subjective symptoms,” according to the Fibromyalgia Rapid Screening Tool (FiRST) questionnaire [42]. Various studies estimate the prevalence of post-Lyme syndrome, as defined, at 5% to 15%. Also, if we consider that one-third of post-Lyme syndrome cases meet the criteria for fibromyalgia, that would mean the prevalence of post-Lyme fibromyalgia is between 2% and 5%, and thus comparable to that of fibromyalgia in developed countries (1% to 4%) [43].
A single-center retrospective study reviewed 48 confirmed cases of Lyme disease in 307 patients. These patients, who were referred for an infectious disease consultation for suspected Lyme disease but had no major criterion for Lyme disease, showed a prevalence of subjective symptoms (asthenia, arthralgia, and myalgia) of 17%, 23%, and 12%, respectively, with significantly lower proportions in the Lyme disease group than the control group. These subjective symptoms were less numerous and less persistent in the Lyme disease group than in the control group, which does not support these symptoms being specifically attributable to Lyme disease, and does not support the argument of a “chronic Lyme” entity. [44].
Most of all, six randomized controlled trials with prolonged antibiotic therapy (3 months) found this therapy to have no health benefit for patients (according to the SF-36 quality of life questionnaire) who fit the definition of post-Lyme syndrome, compared to placebo [45-47].
3.5. Other clinical manifestations
Other rarer manifestations associated with early forms of Lyme disease have been reported: borrelial lymphocytoma (painless swelling, mainly of the earlobe in children, and around the nipple in adults), carditis (atrioventricular block and/or myopericarditis) or ophthalmologic disorders (keratoconjunctivitis, uveitis).
5. Treatment Strategies
The main change to the 2019 French recommendations [14,15] in relation to the 2006 recommendations [27] is the choice of doxycycline as a first-line antibiotic therapy in Lyme disease, regardless of the organ affected (including neuroborreliosis), while the duration depends first and foremost on the time since the infection (Table 2).
Table 2. Treatment of Lyme Disease
| Early Lyme Disease | Late Lyme Disease | |||
| EM | NEUROBORRELIOSIS or other sites | ARTHRITIS | ACA | |
| First-line antibiotic | Doxycycline 100 mg × b.i.d.a | |||
| Duration | 14 days | 28 days | ||
| Other possible antibioticsb | Amoxicillinc Azithromycind Ceftriaxonee Cefuroxime axetilf | Ceftriaxone e | Ceftriaxonee Amoxicillinc | Ceftriaxonee |
EM: Erythema migrans, ACA: Acrodermatitis chronica atrophicans
a one dose of doxycycline 200 mg b.i.d. is preferred in the case of CNS involvement (such as cerebral vascularity, myelitis, or encephalitis).
b therapeutic alternative in the case of a true known allergy to cycline antibiotics, or in children < 8 years of age or viable pregnancy
c the recommended dose of amoxicillin is 1 g t.i.d. (50 mg/kg/day in children)
d the recommended dose of azithromycin is 500 mg per day (20 mg/kg/day in children) over the shortest period of time possible because of significant post-antibiotic effects (EM: 5 days, borrelial lymphocytoma: 10 days)
e the recommended dose of ceftriaxone is 2 g daily (80 mg/kg/in children)
f the recommended dose of cefuroxime axetil is 500 mg b.i.d. (30 mg/kg/day in children). This treatment no longer appears in 2019 French recommendations since azithromycin treatment is preferred in the case of amoxicillin allergy.
5.1. Treatment of EM
Even if the EM improves spontaneously in a few weeks, antibiotic treatment continues to be recommended to prevent other forms of Lyme disease. Doxycycline is the first treatment of choice as it also allows other possible tick-borne co-infections to be eradicated. It requires only 14 days of treatment [15,16].
A meta-analysis reported efficacy comparable to that of other molecules that can be used in the case of contraindications (allergy to cycline antibiotics, children < 8 years of age): amoxicillin, cefuroxime axetil or azithromycin for oral antibiotic therapies. Photosensitivity (3%), digestive problems (10%) and a Jarisch-Herxheimer reaction (15%) have been reported, with no significant difference between the various antibiotics [48].
5.2. Treatment of Lyme arthritis
Lyme arthritis improves spontaneously at one year without antibiotics in only 20% of cases, while 100% will show improvement at five years. Appropriate antibiotic therapy speeds the resolution of Lyme arthritis. The efficacy if IV antibiotic therapy (ceftriaxone) has not been reported to be greater than that of doxycycline. Therefore, the recommended treatment is doxycycline 100 mg b.i.d. for 28 days.
If arthritis persists after this first-line antibiotic therapy, there is little data to justify prolonged antibiotic therapy (3 months) or second-line antibiotic therapy with ceftriaxone. However, it is regularly practiced and continues to be recommended.
Corticosteroid injection of the involved joint is effective and does not seem to increase the risk of recurrence (ARLA). In the case of chronic arthritis that persists in spite of appropriate antibiotic therapy (at least 1 month of doxycycline or ceftriaxone), immunomodulator treatments (hydroxychloroquine or methotrexate) have been suggested; however, they have not been proven to change the natural course of the disease. They could have a symptomatic effect.
5.3. Treatment of neuroborreliosis
Even though doxycycline crosses the blood–brain barrier at a lower rate and does not seem to have any intrathecal antibacterial action [49,50], all of the studies show the noninferiority of doxycycline 100 mg b.i.d. compared with ceftriaxone for neuroborrelioses [51]. Since doxycycline is easier to administer, less likely to cause bacterial resistance, less expensive, and active against several other tick-borne pathogens, it is now the preferred first-line treatment for neuroborrelioses. A short treatment of 14 days was not inferior to a 28-day treatment, or even a 6-week treatment for early forms (< 6 months) [52]. The dose may be doubled to 200 mg b.i.d. if the central nervous system is affected (encephalitis, cerebral vascularity, or myelitis), although this is rarely encountered in rheumatology [10].
5.4. Treatment of ACA
This late form of Lyme disease requires 4 weeks of antibiotic therapy with doxycycline 100 mg b.i.d. The allodynia improves quickly, but the erythematous lesions may take several weeks to resolve, with possible atrophic and sclerotic sequelae [15].
5.5. Prevention of Lyme disease
The treatment of Lyme disease relies on avoiding exposure to the vector: ticks are particularly active from April to October. Mechanical protection is the most effective: wearing shoes, socks, and pants during risky outdoor leisure activities (gardening, walking or sports in wooded areas or tall grass). Repellents applied to the skin are less effective. It is important to carefully inspect the skin after being outdoors to mechanically and quickly remove any ticks using tweezers or a tick removal tool. There is no vaccine for Lyme disease available in France [14].
1The Haute autorité de santé (HAS) is an independent public scientific authority, created by the French Health Insurance Act of 13 August 2004 to improve the quality and sustainability of the French healthcare system. It acts to improve the quality of the healthcare system to ensure that everyone has lasting and equitable access to the most effective, safe, and efficient care possible.
Conflicts of interest: The authors declare that they have no conflict of interest in the data presented in this update.
References
1. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461-73.
2. Keller A, Graefen A, Ball M, Matzas M, Boisguerin V, Maixner F, et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698.
3. Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216(4552):1317–1319.
4. Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977;20(1):7-17.
5. Peretti-Watel P, Ward J, Lutaud R, Seror V. Lyme disease: Insight from social sciences. Med Mal Infect 2019;49(2):133-9.
6. Gocko X, Tattevin P, Lemogne C. Genesis and dissemination of a controversial disease: chronic Lyme. Med Mal Infect. 2020 Oct 9:S0399-077X(20)30726-5. doi: 10.1016/j.medmal.2020.09.026. Epub ahead of print.
7. Stone BL, Tourand Y, Brissette CA. Brave New Worlds: The Expanding Universe of Lyme Disease. Vector Borne Zoonotic Dis. 2017;17(9):619-629.
8. Feng J, Li T, Yee R, Yuan Y, Bai C, Cai M, et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov Med. 2019;27:125–38.
9. Stortenbeker I, Stommel W, van Dulmen S, Lucassen P, Das E, Olde Hartman T. Linguistic and interactional aspects that characterize consultations about medically unexplained symptoms: A systematic review. J Psychosom Res. 2020;132:109994.
10. Bransfield RC, Friedman KJ. Differentiating Psychosomatic, Somatopsychic, Multisystem Illnesses, and Medical Uncertainty. Healthcare (Basel). 2019;7(4):114.
11. Scheerer C, Rüth M, Tizek L, Köberle M, Biedermann T, Zink A. Googling for Ticks and Borreliosis in Germany: Nationwide Google Search Analysis From 2015 to 2018. J Med Internet Res. 2020;22(10):e18581.
12. Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT, Aguero-Rosenfeld ME, Auwaerter PG, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis, and Treatment of Lyme Disease. Arthritis Rheumatol. 2020 Nov 29. doi: 10.1002/art.41562. [full guidelines]
13. IDSA. Public Health. Lyme disease.
14. Figoni J, Chirouze C, Hansmann Y, Lemogne C, Hentgen V, Saunier A, et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): prevention, epidemiology, diagnosis. Med Mal Infect. 2019;49(5):318-334.
15. Jaulhac B, Saunier A, Caumes E, Bouiller K, Gehanno JF, Rabaud C, et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Med Mal Infect. 2019;49(5):335-346.
16. Eldin C, Raffetin A, Bouiller K, Hansmann Y, Roblot F, Raoult D, et al. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med Mal Infect. 2019;49(2):121-132.
17. Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390(6660):580-6.
18. Adeolu M, Gupta RS. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek. 2014;105(6):1049-72.
19. Vandenesch A, Turbelin C, Couturier E, Arena C, Jaulhac B, Ferquel E, et al. Incidence and hospitalisation rates of Lyme borreliosis, France, 2004 to 2012. Euro Surveill 2014;19.
20. Schwameis M, Kündig T, Huber G, von Bidder L, Meinel L, Weisser R, et al. Topical azithromycin for the prevention of Lyme borreliosis: a randomised, placebo-controlled, phase 3 efficacy trial. Lancet Infect Dis. 2017;17(3):322-329.
21. Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345(2):79-84.
22. Nebbak A, Dahmana H, Almeras L, Raoult D, Boulanger N, Jaulhac B, et al. Co-infection of bacteria and protozoan parasites in Ixodes ricinus nymphs collected in the Alsace region, France. Ticks Tick Borne Dis. 2019;10(6):101241.
23. Gilot B, Degeilh B, Pichot J, Doche B, Guiguen C. Prevalence of Borrelia burgdorferi (sensu lato) in Ixodes ricinus (L.) populations in France, according to a phytoecological zoning of the territory. Eur J Epidemiol. 1996;12(4):395-401.
24. Pichon B, Mousson L, Figureau C, Rodhain F, Perez-Eid C. Density of deer in relation to the prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus nymphs in Rambouillet forest, France. Exp Appl Acarol. 1999;23(3):267-75.
25. Quessada T, Martial-Convert F, Arnaud S, Leudet De La Vallee H, Gilot B, Pichot J. Prevalence of Borrelia burgdorferi species and identification of Borrelia valaisiana in questing Ixodes ricinus in the Lyon region of France as determined by polymerase chain reaction-restriction fragment length polymorphism. Eur J Clin Microbiol Infect Dis. 2003;22(3):165-73.
26. World Health Organization, Regional Office for South-East Asia. A brief guide to emerging infectious diseases and zoonoses. WHO Regional Office for South-East Asia. 2014.
27. SPILF. Borréliose de Lyme : démarches diagnostiques, thérapeutiques et préventives. Texte court [Lyme borreliose: diagnostic, therapeutic and preventive approaches. Short text]. Med Mal Infect. 2007;37(4):187-93.
28. Valesová H, Mailer J, Havlík J, Hulínská D, Hercogová J. Long-term results in patients with Lyme arthritis following treatment with ceftriaxone. Infection. 1996;24(1):98-102.
29. Grillon A, Scherlinger M, Boyer PH, De Martino S, Perdriger A, Blasquez A, et al. Characteristics and clinical outcomes after treatment of a national cohort of PCR-positive Lyme arthritis. Semin Arthritis Rheum. 2019;48(6):1105-1112. [manuscript]
30. Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH 3rd, Liu NY, Schmid CH. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37(6):878-88.
31. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-80.
32. Lipowsky C, Altwegg M, Michel BA, Brühlmann P. Detection of Borrelia burgdorferi by species-specific and broad-range PCR of synovial fluid and synovial tissue of Lyme arthritis patients before and after antibiotic treatment. Clin Exp Rheumatol. 2003;21(2):271-2.
33. Bentas W, Karch H, Huppertz HI. Lyme arthritis in children and adolescents: outcome 12 months after initiation of antibiotic therapy. J Rheumatol. 2000;27(8):2025-30.
34. Tory HO, Zurakowski D, Sundel RP. Outcomes of children treated for Lyme arthritis: results of a large pediatric cohort. J Rheumatol. 2010;37(5):1049-55.
35. Horton DB, Taxter AJ, Davidow AL, Groh BP, Sherry DD, Rose CD. Intraarticular Glucocorticoid Injection as Second-line Treatment for Lyme Arthritis in Children. J Rheumatol. 2019;46(8):952-959.
36. Babady NE, Sloan LM, Vetter EA, Patel R, Binnicker MJ. Percent positive rate of Lyme real-time polymerase chain reaction in blood, cerebrospinal fluid, synovial fluid, and tissue. Diagn Microbiol Infect Dis. 2008;62(4):464-6.
37. Miller JB, Aucott JN. Stages of Lyme Arthritis. J Clin Rheumatol. 2020 Aug 17. doi: 10.1097/RHU.0000000000001513. Epub ahead of print.
38. Strle K, Sulka KB, Pianta A, Crowley JT, Arvikar SL, Anselmo A, et al. T-Helper 17 Cell Cytokine Responses in Lyme Disease Correlate With Borrelia burgdorferi Antibodies During Early Infection and With Autoantibodies Late in the Illness in Patients With Antibiotic-Refractory Lyme Arthritis. Clin Infect Dis. 2017;64(7):930-938.
39. Ogrinc K, Lusa L, Lotrič-Furlan S, Bogovič P, Stupica D, Cerar T, Ružić-Sabljić E, Strle F. Course and Outcome of Early European Lyme Neuroborreliosis (Bannwarth Syndrome): Clinical and Laboratory Findings. Clin Infect Dis. 2016;63(3):346- 53.
40. Dupeyron A, Lecocq J, Jaulhac B, Isner-Horobeti ME, Vautravers P, Cohen-Solal J, Sordet C, Kuntz JL. Sciatica, disk herniation, and neuroborreliosis. A report of four cases. Joint Bone Spine. 2004;71(5):433-7.
41. Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089-134.
42. Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Farber S, et al. Long-Term Assessment of Fibromyalgia in Patients with Culture-Confirmed Lyme Disease. Arthritis Rheumatol. 2015;67(3):837-839.
43. Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int. 2017 Sep;37(9):1527-1539.
44. Ranque-Garnier S, Eldin C, Sault C, Raoult D, Donnet A. Management of patients presenting with generalized musculoskeletal pain and a suspicion of Lyme disease. Med Mal Infect. 2019;49(2):157-166.
45. Berende A, Ter Hofstede HJ, Vos FJ, van Middendorp H, Vogelaar ML, Tromp M, et al. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N Engl J Med. 2016;374(13):1209-20.
46. Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345(2):85-92.
47. Sjöwall J, Ledel A, Ernerudh J, Ekerfelt C, Forsberg P. Doxycycline-mediated effects on persistent symptoms and systemic cytokine responses post-neuroborreliosis: a randomized, prospective, cross-over study. BMC Infect Dis. 2012;12:186.
48. Torbahn G, Hofmann H, Rucker G, Bischoff K, Freitag MH, Dersch R, et al. Efficacy and Safety of Antibiotic Therapy in Early Cutaneous Lyme Borreliosis: A Network Meta-analysis. JAMA Dermatol 2018;154:1292–303.
49. Karlsson M, Hammers S, Nilsson-Ehle I, Malmborg AS, Wretlind B. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob Agents Chemother. 1996;40(5):1104-7.
50. Dotevall L, Hagberg L. Penetration of doxycycline into cerebrospinal fluid in patients treated for suspected Lyme neuroborreliosis. Antimicrob Agents Chemother. 1989;33(7):1078-80.
51. Ljøstad U, Skogvoll E, Eikeland R, Midgard R, Skarpaas T, Berg A, et al. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol. 2008;7(8):690- 5. [mirror]
52. Dattwyler RJ, Wormser GP, Rush TJ, Finkel MF, Schoen RT, Grunwaldt E, et al. A comparison of two treatment regimens of ceftriaxone in late Lyme disease. Wien Klin Wochenschr. 2005;117(11-12):393-7.
To cite this version: Guillaume Coiffier, Pierre Tattevin. Lyme disease: ”End of the debate?”. Joint Bone Spine, 2021, 88 (4), pp.105181. 10.1016/j.jbspin.2021.105181 . hal-03372897
© 2021 published by Elsevier. This manuscript is made available under the CC BY NC 4.0 user license.
The LymeScience document repository reformatted the manuscript for internet display.