In North America, after a patient has 30 days of symptoms, the well-established testing protocol for Lyme disease requires both a non-negative ELISA and a Western blot to detect at least 5 of 10 bands.
The 10 bands correspond to different IgG antibodies made by the body in response to infection by B. burgdorferi, the bacterium that causes Lyme disease.
Note: In Europe, there are two additional predominant strains of Lyme disease-causing bacteria, which necessitate different testing.
Because it can take a few weeks for the body to produce detectable IgG antibodies, testing for IgM antibodies may be used in the first 30 days. Unfortunately, IgM testing is known for producing false positives, which is why IgG confirmation is useful if a diagnosis is made using only IgM testing.
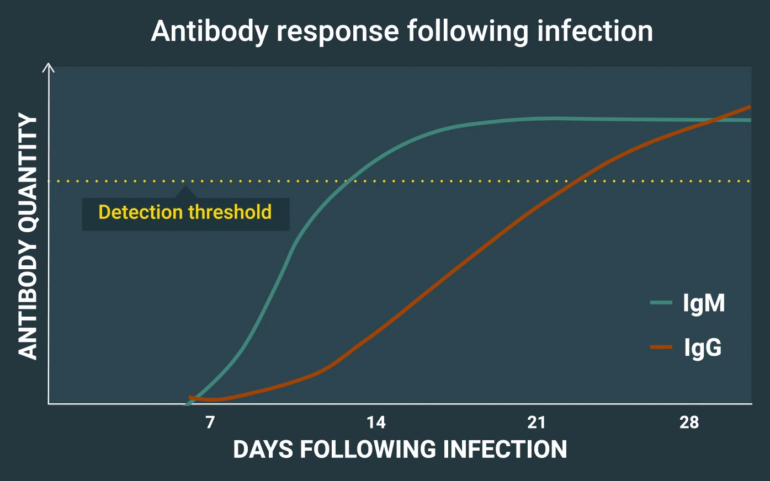
The above illustrative example of antibody production in Lyme disease is from useful CDC videos that explain Lyme disease pre-test probability and serologic testing.
According to the consensus of experts:
Immunoglobulin G (IgG) seronegativity in an untreated patient with months to years of symptoms essentially rules out the diagnosis of Lyme disease, barring laboratory error or a rare humoral immunodeficiency state.
More: from LymeScience: Lyme Disease Tests: Science vs Misconceptions
And no, despite bizarre claims from chronic Lyme pseudoscience activists, no bands were removed from Lyme disease testing standards, including bands 31 and 34.
Sadly, Lyme disease conspiracy theories merged with anti-vaccine conspiracy theories to falsely claim that bands were removed due because of the Lyme disease vaccine, Lymerix . Lymerix was a safe and effective vaccine that has been FDA approved since 1998, but the vaccine manufacturer stopped selling it for business reasons in 2002.
Table of contents
History of Lyme antibody testing
The testing standards for Lyme disease western blots in North America were adopted in 1994 and are well-established.
In the early 1990s, there was an urgent need to standardize because Lyme disease test results were unreliable and varied both within labs and between different labs. The high rates of false negatives and false positives was unacceptable.
The standard for IgG western blots was initially validated in a paper by Dressler and colleagues. The paper was submitted July 15, 1992, revised September 15, 1992, and published in 1993. Prior to publication, the results were partially presented at scientific conferences in November 1991 and May 1992.
The Dressler paper clearly took a lot of time and effort to produce. It describes two major studies:
- A retrospective study of 225 people who either had or did not have Lyme disease, which was used to calculate initial western blotting criteria.
- A prospective study whereby the western blotting criteria were applied to samples from all 237 patients who were seen at a Lyme disease clinic from July 1990 through June 1991.
Retrospective study
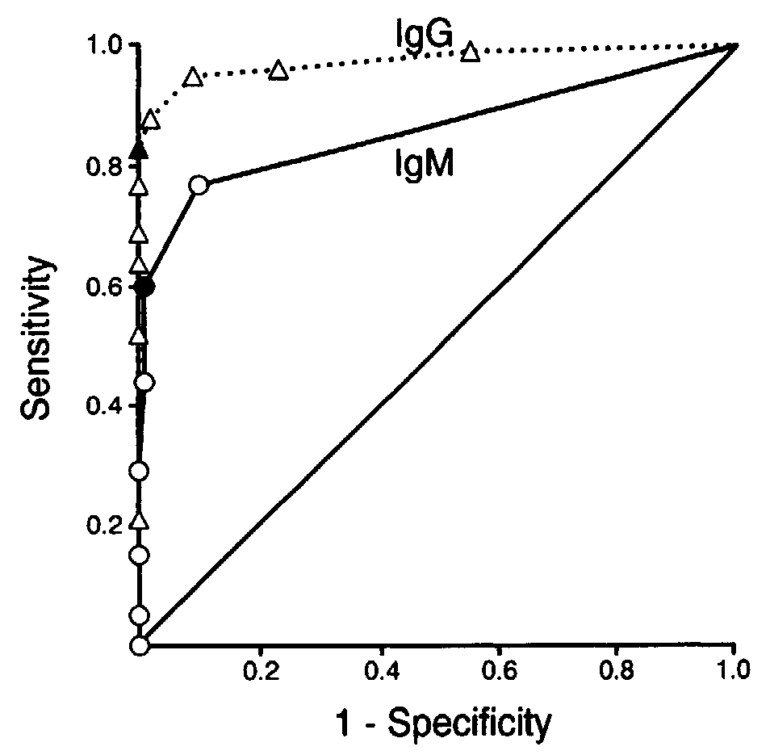
The retrospective study used a standard technique for evaluating diagnostic test performance based on receiver operating characteristics, also known as ROC curves. The greater the area under the curve, the better performance of the test.
The Dressler authors explained their analysis as follows:
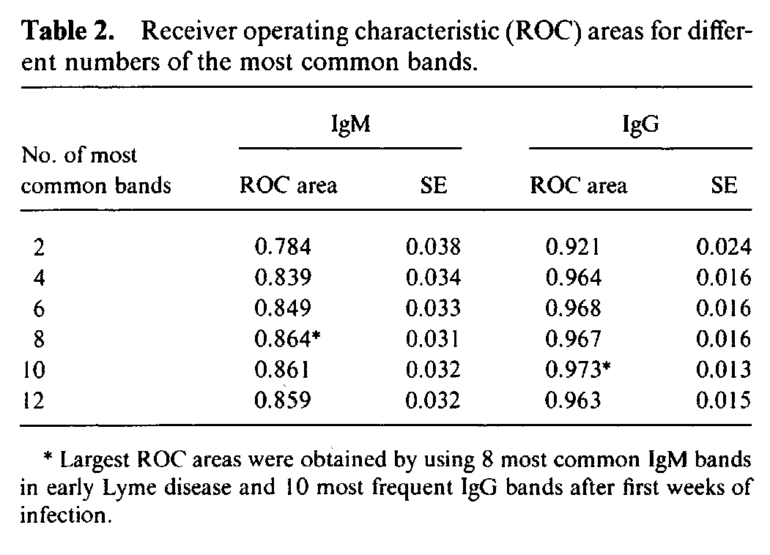
To establish criteria for positive Western blots, we constructed ROC curves for the 13 most common IgM bands in early Lyme disease and for the 14 most frequent IgG bands after the first weeks of infection (table 2).
For IgM blots, the 8 most common bands gave the greatest ROC area of 0.864; for IgG blots, the 10 most frequent bands gave the greatest ROC area of 0.973 (figure 3). Using these optimal ROC curves, we selected the minimum number of IgM or IgG bands needed to obtain 99% specificity.
Thus, in early Lyme disease, at least 2 of the 8 IgM bands at 18, 21, 28, 37, 41, 45, 58, and 93 kDa were required; after the first weeks of infection, at least 5 of the 10 IgG bands at 18, 21, 28, 30, 39, 41, 45, 58, 66, and 93 kDa were needed.
Prospective study
After Dressler and colleagues calculated their diagnostic criteria (2 of 8 positive bands for IgM and 5 of 10 positive bands for IgG), they launched the prospective study to evaluate real-world performance. The prospective study was based on a year of data gathered from all 237 patients who assessed at a Lyme disease clinic from July 1990 through June 1991.
For those interested in the details, we suggest reading the full Dressler paper. But the results of the Dressler prospective study and many other studies have confirmed the usefulness of the Dressler IgG western blotting standard.
What about the IgM western blotting standard?
Peer review and standardization
In October 1994, doctors and scientists gathered at a “National Conference on Serologic Diagnosis of Lyme Disease” co-sponsored by the following agencies:
- The Association of State and Territorial Public Health Laboratory Directors
- Centers for Disease Control and Prevention
- Food and Drug Administration
- National Institutes of Health
- Council of State and Territorial Epidemiologists
- National Committee for Clinical Laboratory Standards (now the Clinical Laboratory Standards Institute)
The experts carefully considered all the data and adopted a two-tier testing standard, with the first tier being an enzyme immunoassay (EIA) or immunofluorescent assay (IFA) followed by a western immunoblot.
The IgG criteria from the Dressler paper were adopted. However, a CDC-sponsored blinded multi-center study compared the Dressler IgM definition (2 of 8 positive IgM bands) to criteria published by Engstrom and colleagues (2 of 3 positive IgM bands). The CDC study found that the Dressler and Engstrom IgM definitions performed similarly, but the Engstrom definition was adopted as the IgM standard because it was simpler and more easily standardized.
Bottom line:
The IgG western blot criteria calculated and verified in the Dressler study were scientifically developed using basic math and are still used today. There is no conspiracy against people who are sold a label of chronic Lyme disease. Chronic Lyme continues to be a pseudoscientific cult and a profitable ecosystem of health fraud, which heavily relies on misinformation.
There is certainly no basis to claims that Lyme disease testing was worsened due to a vaccine or any other reason. It is also a pernicious lie to suggest that Lyme disease testing was developed only for surveillance and not for diagnosing or ruling out Lyme disease.
Standard testing: Useful when used appropriately
Bands 31 and 34 are not among the 10 bands used in standard testing, which is accurate once the body has produced enough antibodies in response to infection. According to the CDC:
- Patients who have had Lyme disease for longer than 4-6 weeks, especially those with later stages of illness involving the brain or the joints, will almost always test positive.
- A patient who has been ill for months or years and has a negative test almost certainly does not have Lyme disease as the cause of their symptoms.
- Serologic testing is generally not useful or recommended for patients with single EM rashes. For this manifestation, a clinical diagnosis (alone) is recommended.
The fallacy of “Special pleading”
According to Dr. Steven Novella:
Special pleading is the process of inventing a special reason to explain away inconvenient evidence or the lack of predicted evidence.
A popular web site about logical fallacies comments:
Humans are funny creatures and have a foolish aversion to being wrong. Rather than appreciate the benefits of being able to change one’s mind through better understanding, many will invent ways to cling to old beliefs. One of the most common ways that people do this is to post-rationalize a reason why what they thought to be true must remain to be true.
It’s usually very easy to find a reason to believe something that suits us, and it requires integrity and genuine honesty with oneself to examine one’s own beliefs and motivations without falling into the trap of justifying our existing ways of seeing ourselves and the world around us.
Special pleading in chronic Lyme groups
In online groups, patients are falsely told that they have Lyme disease based on single positive bands such as band 31, band 34, or band 41. The pseudoscience group ILADS seems to encourage confusion and misdiagnoses by asserting “a positive 31 or 34 band is highly indicative of Borrelia Burgdorferi exposure.”
This is a form of special pleading. Negative tests are ignored because they contradict the beliefs of the group. Most of the bands being negative even after months or years of symptoms is powerful evidence against a Borrelia Burgdoferi infection.
Since bands 31 and 34 almost always accompany other scored bands when a real Borrelia Burgdorferi infection is detected, one or two bands being detected usually indicates a spurious result.
Barbara Johnson, PhD, a scientist with the CDC who specializes in the Lyme disease and serological testing, wrote a very informative chapter in the book “Lyme disease: an evidence-based approach” in which she explains why bands 31 and 34 aren’t used in standardized testing.
The short explanation
Bands 31 and 34 aren’t that useful for predicting Lyme disease. In one study, bands 31 and 34 were only detected in about 15% of the Lyme samples and about 2% of the samples from healthy people.
Also notable is that the band 41 antibody reacted in 43% of the healthy people and 75% of the syphilis patients! That means a test result with a single band 41 is likely not indicative of a Lyme infection.
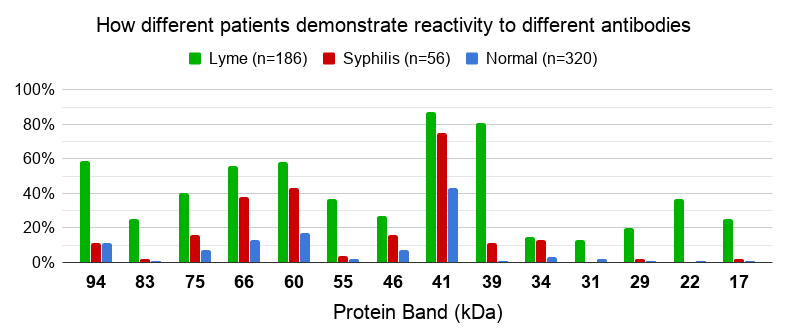
Dr. Johnson’s explanation
4.2.3 Why don’t the scoring criteria for immunoblots include OspA and OspB?
The bands at the 31 and 34 kDa positions of immunoblots are produced by OspA and OspB, respectively (Fig. 4.2). It has been recognized since the early 1990s that antibodies to OspA and OspB are infrequently detected and when they are observed, it is usually in patients with longstanding Lyme arthritis. Ma et al. (1992) wrote that ‘… antibodies against the 31- and 34-kDa proteins were rarely detected and, consequently, became less significant when compared with other protein bands in this study’. Steere’s laboratory reported in Dressler et al. (1993) that, although antibodies to OspA and OspB were detectable in some patients with Lyme arthritis or late neurological disease, the frequency of antibody responses to these polypeptides was not as high as to ten other antigens. Blot interpretation criteria that could best discriminate Lyme disease patients from controls therefore did not include scoring antibodies to OspA or OspB. When bands at 31 or 34 kDa are observed, they are virtually always in the context of a robust IgG response to a large number of scored antigens.
Patients may inquire specifically about why OspA is not scored when it was the basis for an effective vaccine. People naturally think of the usual way that vaccines work, neutralizing infection in a mammalian host, and expect a vaccine antigen to be a good diagnostic antigen. They may be unaware that the OspA vaccine works by killing B. burgdorferi in vector ticks as they feed. OspA is well expressed by B. burgdorferi in unfed ticks and is a suitable target for antibodies that enter a tick during a blood meal from an OspA-vaccinated host. When ticks are exposed to a blood meal and the body temperature of a mammal, B. burgdorferi stops expressing OspA. Another outer-surface protein, OspC, is expressed instead. Reciprocal expression of these two Osps has been demonstrated at the level of single cells. It is not surprising, therefore, that antibody responses to OspC are diagnostically useful in early Lyme disease, but responses to OspA are lacking.
In later manifestations of Lyme disease, especially Lyme arthritis, some people develop antibodies to OspA and/or OspB. OspA expression is upregulated in an inflammatory milieu such as an arthritic joint. OspA expression can be artificially upregulated in a controlled in vivo environment by exposure to zymosan, a yeast cell-wall extract that induces inflammation. Thus, it is no longer a paradox that B. burgdorferi expresses little or no OspA as it is transmitted to mammalian hosts, but that OspA can be produced late in the course of untreated Lyme disease.
Some claim that patients should be judged seropositive based on finding immunoblot bands solely at the 31 or 34 kDa positions, even when their serum is negative by an ELISA that uses whole-cell antigens. However, B. burgdorferi grown in culture expresses OspA and OspB abundantly and ELISAs made from cultured whole cells contain these antigens. Thus, samples from patients who have diagnostically significant levels of antibodies to OspA or OspB will react in a whole-cell-lysate ELISA. When an ELISA is negative but an immunoblot of the same sample is scored positive, it is probable that faint immunoblot bands are being ‘over-read’.
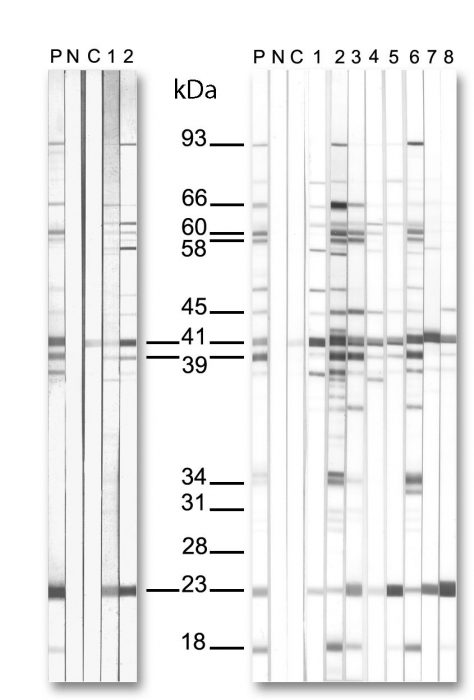
Fig. 4.2. Examples of conventional IgM (left panel) and IgG (right panel) immunoblots. Bands that are recommended for scoring* are labelled. Two additional bands in the IgG blot are also labelled (OspA and OspB at 31 and 34 kDa, respectively; see text). Blots are considered to be positive if two of the three indicated IgM bands or five of the ten indicated IgG bands (excluding OspA and OspB) are present at an intensity equal to or greater than the calibration control.
Left panel: IgM blot profiles for a patient with acute EM (lane 1) and for the same patient at convalescence (lane 2). Note the increase in the number and intensity of the bands at convalescence.
Right panel: IgG blot profiles for eight patients with later manifestations of Lyme borreliosis (lanes 1–8). P, Positive-control serum; N, negative-control serum; C, calibration control (weak positive control). The molecular mass is indicated (kDa). The calibration controls (weak positive controls) have been digitally enhanced for greater clarity in reproduction.
Dr. Alan Barbour also comments:
With the exception of patients with late-stage pauciarticular arthritis, seropositive individuals with B. burgdorferi infection seldom have detectable antibodies to these proteins. At least one reference laboratory includes antibodies to OspA and OspB in their set of proteins for interpretation of Western blot bands of whole cell lysates, but in my experience the high concentration of these acidic proteins on the membranes leads to false-positive bands at 31 kDa and/or 34 kDa.
The scored IgG bands are 18 kDa, 24 kDa (OspC), 28 kDa, 30 kDa, 39 kDa (BmpA), 41 kDa flagellin (Fla), 45 kDa, 58 kDa (not GroEL), 66 kDa, and 93 kDa. The scored IgM bands are 24 kDa (OspC), 39 kDa (BmpA), and 41 kDa (Fla)
The figure above uses band 23 for OspC. According to the CDC, “Depending upon the assay, OspC could be indicated by a band of 21, 22, 23, 24 or 25 kDA.”
Resources
Full chapter: “Laboratory Diagnostic Testing for Borrelia burgdorferi Infection” by Barbara J.B. Johnson, Phd, a scientist at the CDC
CDC: Lyme Diagnosis and Testing
CDC: Laboratory tests that are not recommended
CDC video: Pretest Probability of Lyme disease
CDC video: Lyme disease testing
Updated August 6, 2024